TRIPROLIDINE HYDROCHLORIDE- triprolidine hydrochloride liquid
Woodward Pharma Services LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
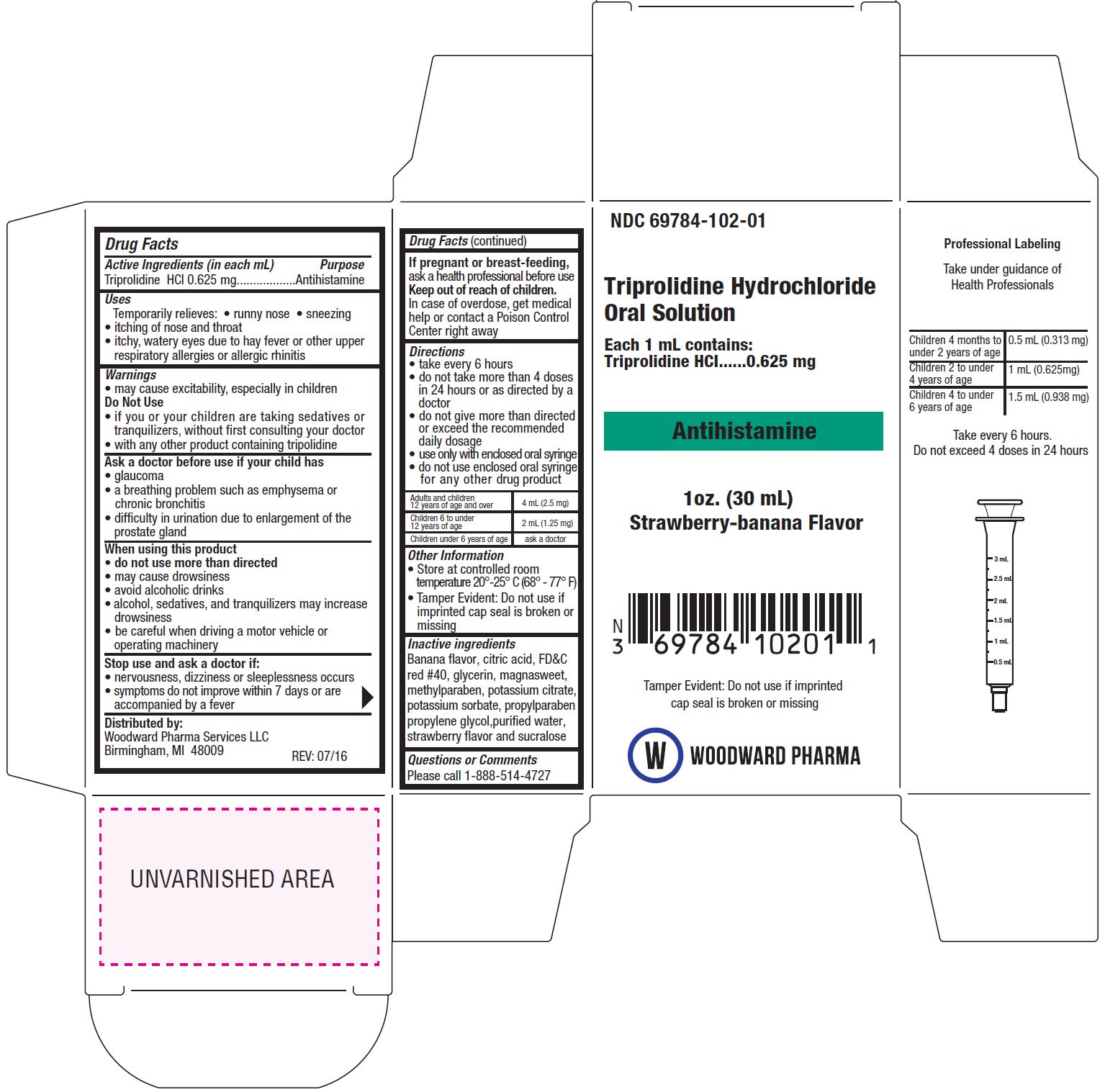
Triprolidine Hydrochloride
Uses
- Temporarily relieves:
- runny nose
- sneezing
- itching of nose and throat
- itchy, watery eyes due to hay fever or other upper respiratory allergies or allergic rhinitis
Warnings
- may cause excitability, especially in children
Do Not Use
- if you or your children are taking sedatives or tranquilizers, without first consulting your doctor
- with any other product containing triprolidine
Ask a doctor before use if your child has
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
When using this product
- do not use more than directed
- may cause drowsiness
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
Directions
- take every 6 hours
- do not take more than 4 doses in 24 hours or as directed by a doctor
- do not give more than directed or exceed the recommended daily dosage
- use only with enclosed oral syringe
- do not use enclosed oral syringe for any other drug product
| Adults and children 12 years of age and over | 4 mL (2.5 mg) | |
| Children 6 to under 12 years of age | 2 mL (1.25 mg) | |
| Children under 6 years of age | ask a doctor |
Other Information
- store at controlled room temperature 20°-25°C (68°-77°F)
- Tamper Evident: Do not use if seal is broken or missing.
Inactive ingredients
Banana flavor, citric acid, FD&C red #40, glycerin, magnasweet, methylparaben, potassium citrate, potassium sorbate, propylparaben, propylene glycol, purified water, strawberry flavor and sucralose
Questions or Comments
Please Call 1-888-514-4727
Distributed by:
Woodward Pharma Services LLC
Birmingham, MI 48009
| TRIPROLIDINE HYDROCHLORIDE
triprolidine hydrochloride liquid |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Woodward Pharma Services LLC (026749066) |
Revised: 6/2020
Document Id: bee5cde2-9763-41ba-a061-9bf8216c1487
Set id: 5c2fb4cc-c5a3-421c-91d4-c5bbe2189467
Version: 4
Effective Time: 20200601
Woodward Pharma Services LLC