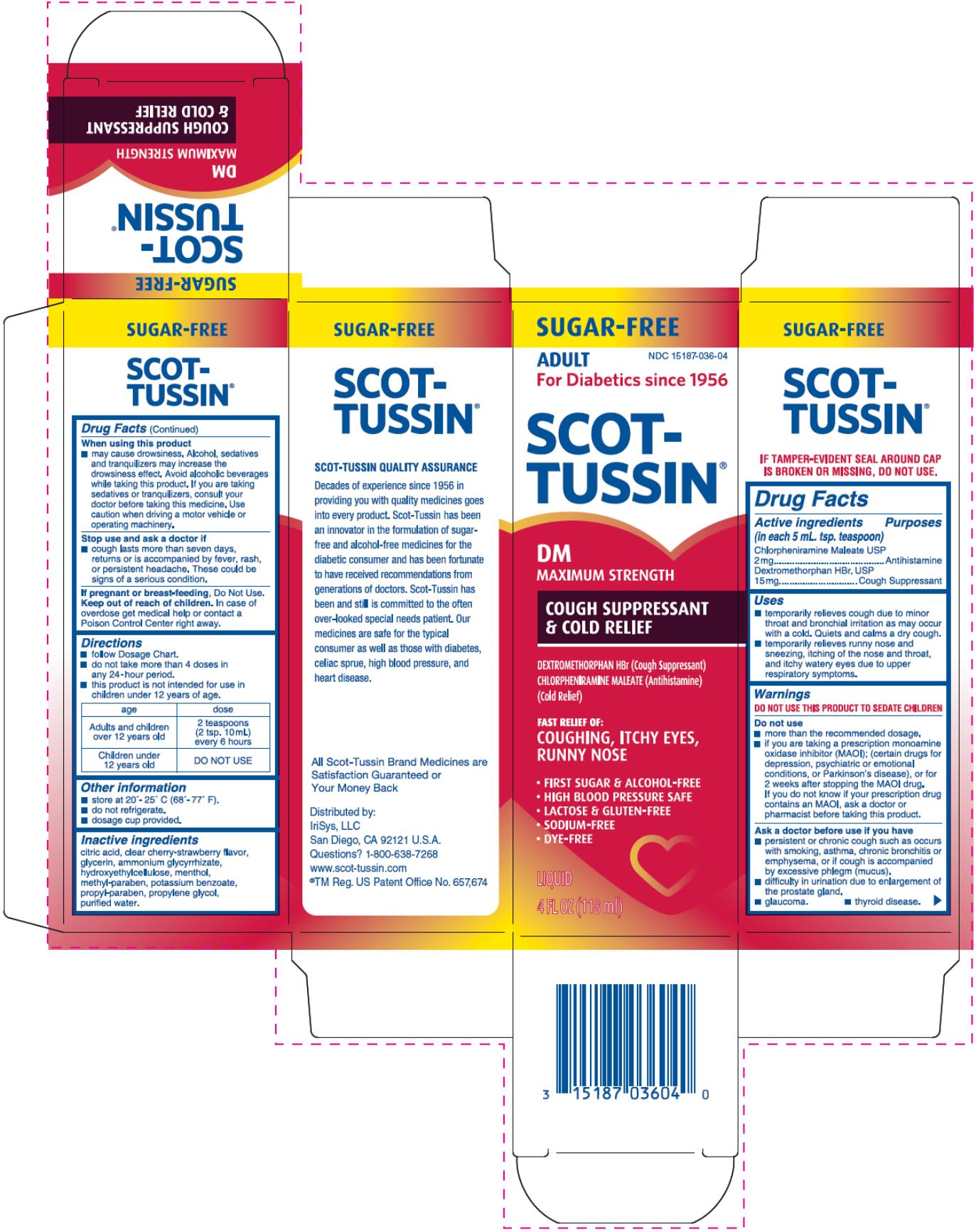
SCOT-TUSSIN DM MAXIMUM STRENGTH COUGH SUPPRESSANT AND COLD RELIEF- chlorpheniramine maleate and dextromethorphan hydrobromide liquid
SOCIETAL CDMO SAN DIEGO, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Scot-Tussin DM Maximum Strength Cough Suppressant and Cold Relief
Uses
- temporarily relieves cough due to minor throat and bronchial irritation as may occur with cold. Quiets and calms a dry cough.
- temporarily relieves runny nose and sneezing, itching of the nose and throat, and itchy watery eyes due to upper respiratory symptoms.
Warnings
DO NOT USE THIS PRODUCT TO SEDATE CHILDREN
Do not use:
- more than the recommended dosage.
- if you are taking a prescription monoamine oxidase inhibitor (MAOI); (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis or emphysema, or if cough is accompanied by excessive phlegm (mucus).
- difficulty in urination due to enlargement of the prostate gland.
- glaucoma.
- thyroid disease.
When using this product
- may cause drowsiness. Alcohol, sedatives and tranquilizers may increase the drowsiness effect. Avoid alcoholic beverages while taking this product. If you are taking sedatives or tranquilizers, consult your doctor before taking this medicine. Use caution when driving a motor vehicle or operating machinery.
Directions
- follow Dosage Chart.
- do not take more than 4 doses in any 24-hour period.
- this product is not intended for use in children under 12 years of age.
| age | dose |
| Adults and children over 12 years old | 2 teaspoons (2 tsp. 10 ml) every 6 hours |
| Children under 12 years old | DO NOT USE |
Inactive ingredients
citric acid, clear cherry-strawberry flavor, glycerin, ammonium glycyrrhizate, hydroxyethylcellulose, menthol, methyl-paraben, potassium benzoate, propyl-paraben, propylene glycol, purified water.
Distributed by:
IriSys, LLC
San Diego, CA 92121 U.S.A.
Questions? 1-800-638-7268
www.scot-tussin.com
®TM Reg. US Patent Office No. 657, 674
PRINCIPAL DISPLAY PANEL
SUGAR-FREE
ADULT
NDC 15187-036-04
For Diabetics since 1956
SCOT-TUSSIN
®
DM
MAXIMUM STRENGTH
COUGH SUPPRESSANT
& COLD RELIEF
DEXTROMETHORPHAN HBr (Cough Suppressant)
CHLORPHENIRAMINE MALEATE (Antihistamine)
(Cold Relief)
FAST RELIEF OF:
COUGHING, ITCHY EYES, RUNNY NOSE
- FIRST SUGAR & ALCOHOL-FREE
- HIGH BLOOD PRESSURE SAFE
- LACTOSE & GLUTEN-FREE
- SODIUM-FREE
- DYE-FREE
LIQUID
4 FL OZ (118 ml)
| SCOT-TUSSIN DM MAXIMUM STRENGTH COUGH SUPPRESSANT AND COLD RELIEF
chlorpheniramine maleate and dextromethorphan hydrobromide liquid |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - SOCIETAL CDMO SAN DIEGO, LLC (079682716) |