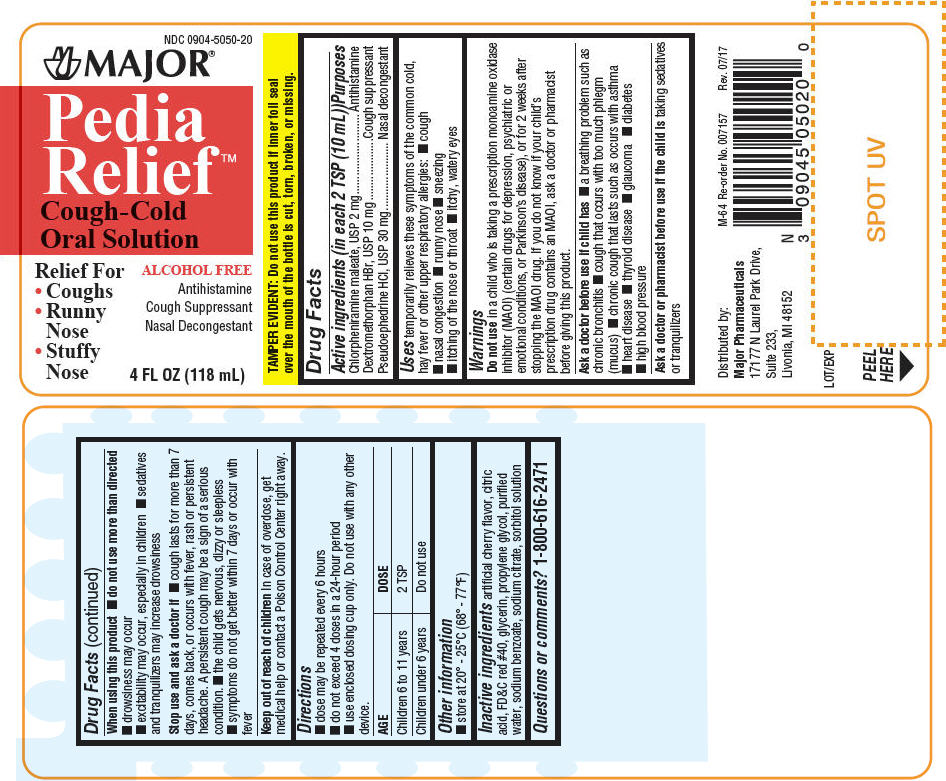
PEDIA RELIEF- chlorpheniramine maleate and pseudoephedrine hydrochloride and dextromethorphan hydrobromide liquid
Major Pharmaceuticals, Inc
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
| Active ingredients (in each 2 TSP (10 mL)) | Purposes |
| Chlorpheniramine maleate, USP 2 mg | Antihistamine |
| Dextromethorphan HBr, USP 10 mg | Cough suppressant |
| Pseudoephedrine HCl, USP 30 mg | Nasal decongestant |
Uses
temporarily relieves these symptoms of the common cold, hay fever or other upper respiratory allergies:
- cough
- nasal congestion
- runny nose
- sneezing
- itching of the nose or throat
- itchy, watery eyes
Warnings
Do not use in a child who is taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your child's prescription drug contains an MAOI, ask a doctor or pharmacist before giving this product.
Ask a doctor before use if child has
- a breathing problem such as chronic bronchitis
- cough that occurs with too much phlegm (mucus)
- chronic cough that lasts such as occurs with asthma
- heart disease
- thyroid disease
- glaucoma
- diabetes
- high blood pressure
Ask a doctor or pharmacist before use if the child is taking sedatives or tranquilizers
When using this product
-
do not use more than directed
- drowsiness may occur
- excitability may occur, especially in children
- sedatives and tranquilizers may increase drowsiness
Stop use and ask a doctor if
- cough lasts for more than 7 days, comes back, or occurs with fever, rash or persistent headache. A persistent cough may be a sign of a serious condition.
- the child gets nervous, dizzy or sleepless
- symptoms do not get better within 7 days or occur with fever
Keep out of reach of children In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- dose may be repeated every 6 hours
- do not exceed 4 doses in a 24-hour period
- use enclosed dosing cup only. Do not use with any other device.
| AGE | DOSE |
| Children 6 to 11 years | 2 TSP |
| Children under 6 years | Do not use |
Other information
- store at 20° - 25°C (68° - 77°F)
Inactive ingredients
artificial cherry flavor, citric acid, FD&C red #40, glycerin, propylene glycol, purified water, sodium benzoate, sodium citrate, sorbitol solution
Questions or comments?
1-800-616-2471
Distributed by:
Major Pharmaceuticals
17177 N Laurel Park Drive,
Suite 233,
Livonia, MI 48152
PRINCIPAL DISPLAY PANEL - 118 mL Bottle Label
NDC 0904-5050-20
MAJOR®
Pedia
Relief
™
Cough-Cold
Oral Solution
Relief For
-
Coughs
-
Runny
Nose
-
Stuffy
Nose
ALCOHOL FREE
Antihistamine
Cough Suppressant
Nasal Decongestant
4 FL OZ (118 mL)