HEMORRHOIDS- calcium fluoride,strychnos nux-vomica seed,horse chestnut and krameria lappacea root tablet
Hyland's
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Hemorrhoids
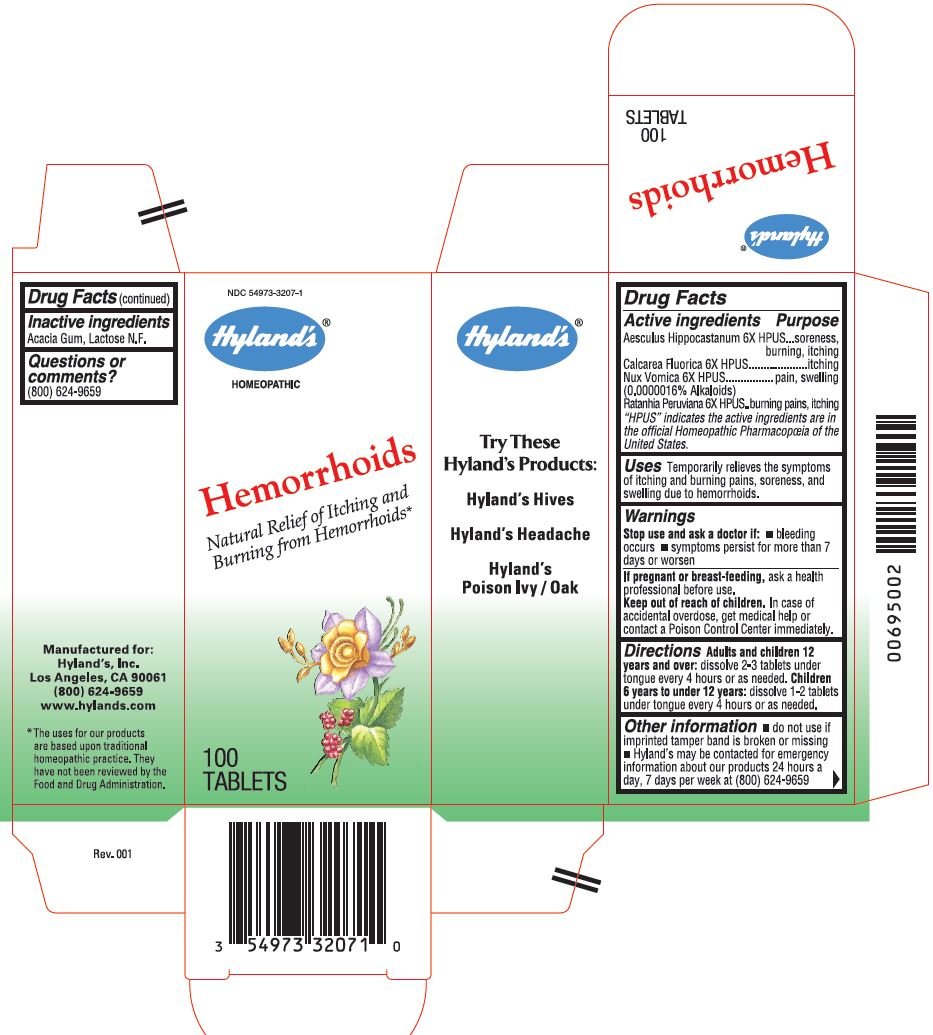
Drug Facts
| Active ingredients | Purpose |
| Aesculus Hippocastanum 6X HPUS | soreness, burning,itching |
| Calcarea Fluorica 6X HPUS | itching |
|
Nux Vomica 6X HPUS (0.0000016% Alkaloids) | pain, swelling |
| Ratanhia Peruviana 6X HPUS | burning pains, itching |
"HPUS" indicates the active ingredients are in the official Homeopathic Pharmacopœia of the United States.
Uses
Temporarily relieves the symptoms of itching and burning pains, soreness, and swelling due to hemorrhoids.
Directions
Adults and children 12 years and over: dissolve 2-3 tablets under tongue every 4 hours or as needed.
Children 6 years to under 12 years: dissolve 1-2 tablets under tongue every 4 hours or as needed.
| HEMORRHOIDS
calcium fluoride,strychnos nux-vomica seed,horse chestnut and krameria lappacea root tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Hyland's (028570695) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Standard Homeopathic Company | 008316655 | manufacture(54973-3207) , pack(54973-3207) , label(54973-3207) | |