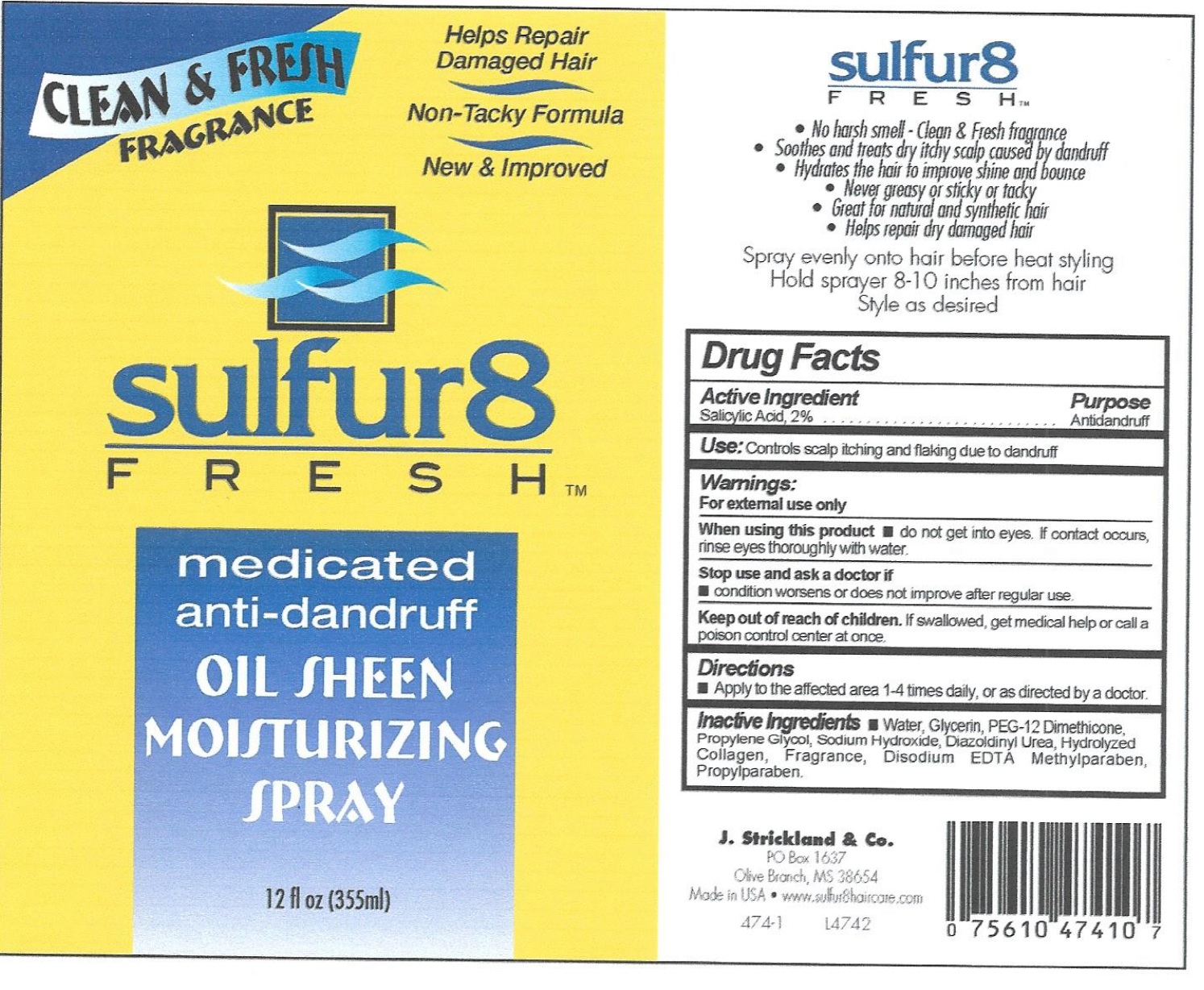
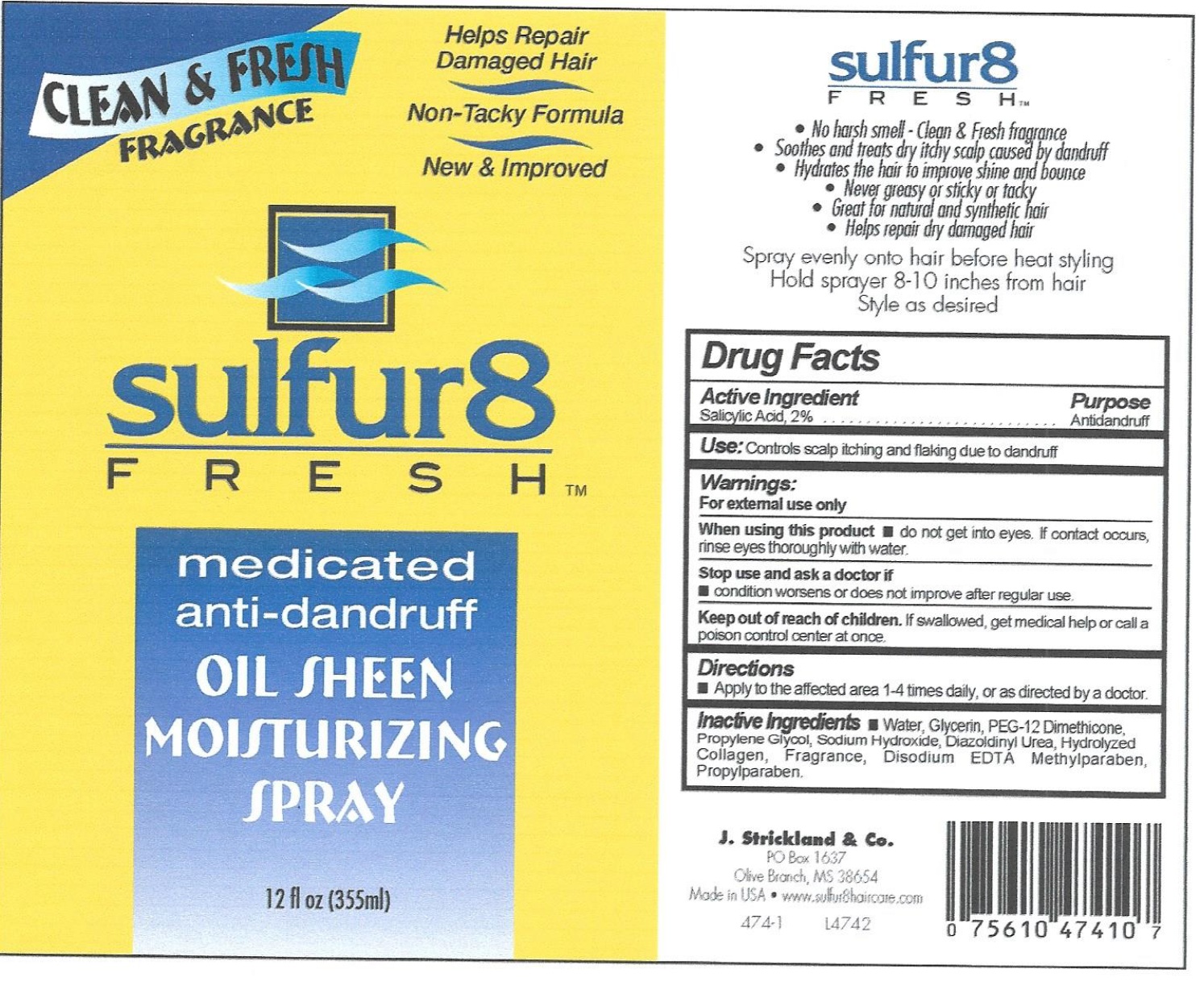
Label: SULFUR 8 FRESH ANTI-DANDRUFF- salicylic acid solution
- NDC Code(s): 12022-028-00
- Packager: J. Strickland & Co.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Use:
- Warnings:
- Directions
-
Inactive Ingredients
water, Mineral Oil, Aloe Barbadensis Leaf Juice, Isopropyl Myristate, Stearyl Alcohol, Ceteareth-20, Sodium Carbomer, Simmondsia Chinensis (Jojoba) Seed Oil, Hydrolyzed Collagen, Propylene Glycol, Tocopheryl Acetate, Methylparaben, Propylparaben, Diazolidinyl Urea, Sodium Lauryl Sulfate, Panthenol, Fragrance.
- Package Labeling
-
INGREDIENTS AND APPEARANCE
SULFUR 8 FRESH ANTI-DANDRUFF
salicylic acid solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:12022-028 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PEG-12 DIMETHICONE (UNII: ZEL54N6W95) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM HYDROXIDE (UNII: 55X04QC32I) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:12022-028-00 355 mL in 1 BOTTLE; Type 0: Not a Combination Product 02/06/2008 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 02/06/2008 Labeler - J. Strickland & Co. (007023112) Registrant - J. Strickland & Co. (007023112) Establishment Name Address ID/FEI Business Operations J. Strickland & Co. 007023112 manufacture(12022-028)