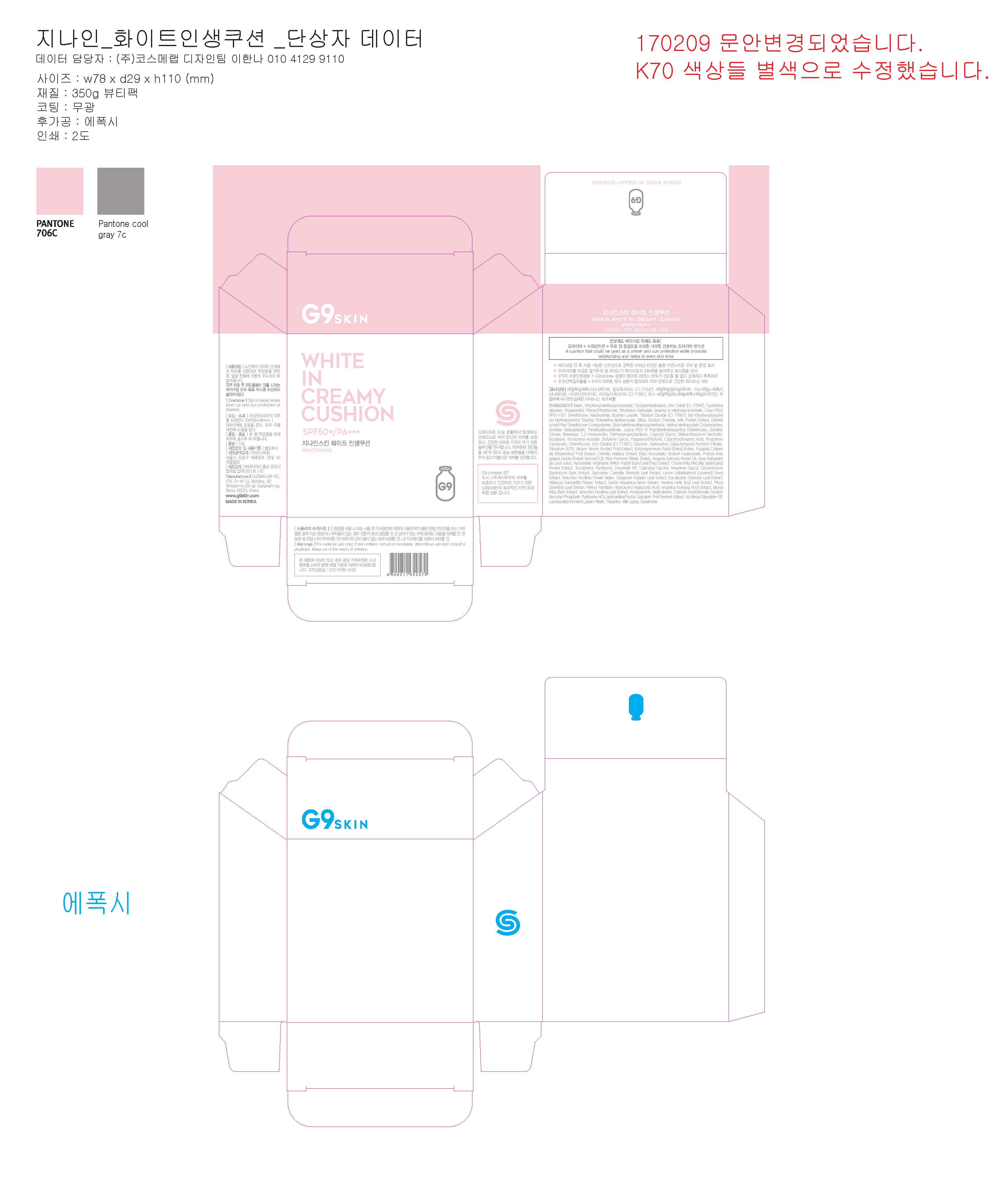
G9 SKIN WHITE IN CREAMY CUSHION SPF50 PA- titanium dioxide powder
COSMELAB Co., Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Drug Facts
Water, Ethylhexyl Methoxycinnamate, Cyclopentasiloxane, Zinc Oxide (CI 77947), Cyclohexasiloxane, Propanediol, Ethylhexyl Salicylate, Isoamyl p-Methoxycinnamate, Phenyl Trimethicone, Cetyl PEG/PPG-10/1 Dimethicone, Niacinamide, Isoamyl Laurate, Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine, Polymethyl Methacrylate, Silica, Sodium Chloride, Dimethicone/Vinyl Dimethicone Crosspolymer, Butyl Methoxydibenzoylmethane, Methyl Methacrylate Crosspolymer, Sorbitan Sesquioleate, Trimethylsiloxysilicate, Lauryl PEG-9 Polydimethylsiloxyethyl Dimethicone, Sorbitan Olivate, Beeswax, 1,2-Hexanediol, Triethoxycaprylylsilane, Caprylyl Glycol, Disteardimonium Hectorite, Tocopheryl Acetate, Squalane, Fragrance(Parfum), Butylene Glycol, Caprylhydroxamic Acid, Propylene Carbonate, Adenosine, Dimethicone, Disodium EDTA, Illicium Verum (Anise) Fruit Extract, Iron Oxides (CI 77491), Butyrospermum Parkii (Shea) Butter, Morus Alba Bark Extract, Camellia Sinensis Leaf Extract, Linum Usitatissimum (Linseed) Seed Extract, Nelumbo Nucifera Flower Water, Origanum Vulgare Leaf Extract, Eucalyptus Globulus Leaf Extract, Hibiscus Sabdariffa Flower Extract, Salvia Hispanica Seed Extract, Hedera Helix (Ivy) Leaf Extract, Pinus Sylvestris Leaf Extract, Fragaria Chiloensis (Strawberry) Fruit Extract, Lactobacillus/Punica Granatum Fruit Ferment Extract, Retinyl Palmitate, Centella Asiatica Extract, Butyl Avocadate, Sodium Hyaluronate, Prunus Amygdalus Dulcis (Sweet Almond) Oil, Rice Ferment Filtrate (Sake), Argania Spinosa Kernel Oil, Aloe Barbadensis Leaf Juice, Milk Protein Extract, Hamamelis Virginiana (Witch Hazel) Bark/Leaf/Twig Extract, Chamomilla Recutita (Matricaria) Flower Extract, Tocopherol, Panthenol, Hydrolyzed Hyaluronic Acid, Ceramide NP, Galactomyces Ferment Filtrate, Angelica Koreana Root Extract, Nelumbo Nucifera Leaf Extract, Capryloyl Glycine, Hexylene Glycol, Amylodextrin, Glycerin, Maltodextrin, Calcium Pantothenate, Sarcosine, Sodium Ascorbyl Phosphate, Cinnamomum Zeylanicum Bark Extract, Pyridoxine HCl, Theanine, Milk Lipids, Nicotinoyl Dipeptide-26, Glutathione, Lactobacillus Ferment Lysate Filtrate
This "cushion" can be used as a primer and as sunscreen (SPF50+/PA+++), but also moisturizes and brightens your skin
Soak the pad with some product and apply to the desired areas by gently tapping the pad against the skin
Cautions :
1. When you use this product and find any of following problems, stop using it. if you keep using it despite the problem, the symptom will get worse, so you will have to go see a dermatologist.
- During use, if you have red spots, swelling, itching, or any other stimuli.
- if the applied region has any problem above for direct light.
2. Do not use the product on wound or dermatitis. 3.Attentive points for storage and treatment :
- Store it in the place where infants or children cannot touch
- do not store it where it has either high or low temperature or there is direct light.
| G9 SKIN WHITE IN CREAMY CUSHION SPF50 PA
titanium dioxide powder |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - COSMELAB Co., Ltd. (688211711) |
| Registrant - COSMELAB Co., Ltd. (688211711) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| COSMELAB Co., Ltd. | 688211711 | label(71790-001) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cit Co., Ltd | 690081646 | manufacture(71790-001) | |