Label: DICYCLOMINE- dicyclomine hydrochloride tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 16590-324-30, 16590-324-60, 16590-324-72, 16590-324-90 - Packager: STAT RX USA LLC
- This is a repackaged label.
- Source NDC Code(s): 0143-1227
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 27, 2009
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Dicyclomine hydrochloride is an antispasmodic and anticholinergic (antimuscarinic) agent available in the following form:
Each tablet, for oral administration, contains 20 mg of dicyclomine hydrochloride. They also contain the following inactive ingredients: Anhydrous Lactose, FDandC Blue No. 1 Lake, Lactose Monohydrate, Magnesium Stearate, and Microcrystalline Cellulose.
Chemically, dicyclomine hydrochloride is [bicyclohexyl]-1-carboxylic acid, 2-(diethylamino) ethylester, hydrochloride with the structural formula: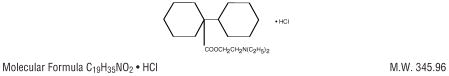 DICYCLOMINE 20MG STRUCTURE IMAGE
DICYCLOMINE 20MG STRUCTURE IMAGEDicyclomine hydrochloride occurs as a fine, white, crystalline, practically odorless powder with a bitter taste. It is soluble in water, freely soluble in alcohol and chloroform, and very slightly soluble in ether
-
CLINICAL PHARMACOLOGY
Dicyclomine relieves smooth muscle spasm of the gastrointestinal tract. Animal studies indicate that this action is achieved via a dual mechanism: (1) a specific anticholinergic effect (antimuscarinic) at the acetylcholine-receptor sites with approximately 1/8 the milligram potency of atropine (in vitro, guinea pig ileum); and (2) a direct effect upon smooth muscle (musculotropic) as evidenced by dicyclomine's antagonism of bradykinin- and histamine-induced spasms of the isolated guinea pig ileum. Atropine did not affect responses to these two agonists. In vivo studies in cats and dogs showed dicyclomine to be equally potent against acetylcholine (ACh)- or barium chloride (BaCl2)-induced intestinal spasm while atropine was at least 200 times more potent against effects of ACh than BaCl2. Tests for mydriatic effects in mice showed that dicyclomine was approximately 1/500 as potent as atropine; antisialagogue tests in rabbits showed dicyclomine to be 1/300 as potent as atropine.
In man, dicyclomine is rapidly absorbed after oral administration, reaching peak values within 60-90 minutes. The principal route of elimination is via the urine (79.5% of the dose). Excretion also occurs in the feces, but to a lesser extent (8.4%). Mean half-life of plasma elimination in one study was determined to be approximately 1.8 hours when plasma concentrations were measured for 9 hours after a single dose. In subsequent studies, plasma concentrations were followed for up to 24 hours after a single dose, showing a secondary phase of elimination with a somewhat longer half-life. Mean volume of distribution for a 20 mg oral dose is approximately 3.65 L/kg suggesting extensive distribution in tissues.
In controlled clinical trials involving over 100 patients who received drug, 82% of patients treated for functional bowel/irritable bowel syndrome with dicyclomine hydrochloride at initial doses of 160 mg daily (40 mg q.i.d.) demonstrated a favorable clinical response compared with 55% treated with placebo. (P less than .05). In these trials, most of the side effects were typically anticholinergic in nature (see table) and were reported by 61% of the patients.
Dicyclomine Hydrochloride
Side (40 mg q.i.d.) Placebo
Effect % %
Dry Mouth 33 5
Dizziness 29 2
Blurred Vision 27 2
Nausea 14 6
Light-Headedness 11 3
Drowsiness 9 1
Weakness 7 1
Nervousness 6 2
Nine percent (9%) of patients were discontinued from the drug because of one or more of these side effects (compared with 2% in the placebo group). In 41% of the patients with side effects, side effects disappeared or were tolerated at the 160 mg daily dose without reduction. A dose reduction from 160 mg daily to an average daily dose of 90 mg was required in 46% of the patients with side effects who then continued to experience a favorable clinical response; their side effects either disappeared or were tolerated. (See ADVERSE REACTIONS.) - INDICATIONS AND USAGE
-
CONTRAINDICATIONS
1. Obstructive uropathy
2. Obstructive disease of the gastrointestinal tract
3. Severe ulcerative colitis (See PRECAUTIONS)
4. Reflux esophagitis
5. Unstable cardiovascular status in acute hemorrhage
6. Glaucoma
7. Myasthenia gravis
8. Evidence of prior hypersensitivity to dicyclomine hydrochloride or other ingredients of these formulations
9. Infants less than 6 months of age (See WARNINGS and PRECAUTIONS: Information for Patients.)
10. Nursing Mothers (See WARNINGS and PRECAUTIONS: Information for Patients.)
-
WARNINGS
In the presence of a high environmental temperature, heat prostration can occur with drug use (fever and heat stroke due to decreased sweating). If symptoms occur, the drug should be discontinued and supportive measures instituted.
Diarrhea may be an early symptom of incomplete intestinal obstruction, especially in patients with ileostomy or colostomy. In this instance, treatment with this drug would be inappropriate and possibly harmful.
Dicyclomine hydrochloride may produce drowsiness or blurred vision. The patient should be warned not to engage in activities requiring mental alertness, such as operating a motor vehicle or other machinery or performing hazardous work while taking this drug.
Psychosis has been reported in sensitive individuals given anticholinergic drugs. CNS signs and symptoms include confusion, disorientation, short-term memory loss, hallucinations, dysarthria, ataxia, coma, euphoria, decreased anxiety, fatigue, insomnia, agitation and mannerisms, and inappropriate affect. These CNS signs and symptoms usually resolve within 12 to 24 hours after discontinuation of the drug.
DICYCLOMINE IS CONTRAINDICATED IN INFANTS LESS THAN 6 MONTHS OF AGE AND IN NURSING MOTHERS. (See CONTRAINDICATIONS and PRECAUTIONS: Nursing Mothers and Pediatric Use).
Safety and efficacy of dicyclomine hydrochloride in children have not been established. -
HOW SUPPLIED
Dicyclomine Hydrochloride Tablets USP, 20 mg are supplied as blue, round, unscored tablets; embossed “WW 27” and are available in:
Bottles of 100 tablets.
Bottles of 1000 tablets.
Unit Dose Boxes of 100 tablets.
To prevent fading, avoid exposure to direct sunlight. Store at 20°-25°C (68°-77°F) [See USP Controlled Room Temperature]. Protect from light and moisture.
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
Manufactured By:
West-ward Pharmaceutical Corp.
Eatontown, NJ 07724
Revised March 200 - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DICYCLOMINE
dicyclomine hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:16590-324(NDC:0143-1227) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DICYCLOMINE HYDROCHLORIDE (UNII: CQ903KQA31) (DICYCLOMINE - UNII:4KV4X8IF6V) DICYCLOMINE HYDROCHLORIDE 20 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) Product Characteristics Color blue Score no score Shape ROUND Size 7mm Flavor Imprint Code WW;27 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:16590-324-30 30 in 1 BOTTLE, PLASTIC 2 NDC:16590-324-60 60 in 1 BOTTLE, PLASTIC 3 NDC:16590-324-90 90 in 1 BOTTLE, PLASTIC 4 NDC:16590-324-72 120 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA040161 10/01/1996 Labeler - STAT RX USA LLC (786036330) Establishment Name Address ID/FEI Business Operations STAT RX USA LLC 786036330 repack, relabel


 DICYCLOMINE 20MG LABEL IMAGE
DICYCLOMINE 20MG LABEL IMAGE