Label: LIDOCARE ARM, NECK, LEG- 4% lidocaine patch
- NDC Code(s): 69993-450-06
- Packager: NFI, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 3, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Use
-
Warnings
For external use only.
Do not use if pouch is damaged or opened. Do not use
- •
- on large areas of the body or on cut, irritated skin
- •
- on open wounds or sensitive skin
- •
- for more than one week without consulting a doctor
- •
- do not use if you are allergic to lidocaine or any of the inactive ingredients listed below.
When using this product
- •
- use only as directed. Read and follow all directions and warnings on this package.
- •
- do not allow contact with the eyes
- •
- do not bandage or apply local heat (such as heating pads) to the area of use
- •
- do not reuse patch.
-
Directions
Adults and children over 12 years of age: Apply patch to affected area every 8 to 12 hours.
Children 12 years or younger: Consult a physician before use.Instructions for Use
- •
- clean and dry affected area
- •
- open pouch and remove the patch
- •
- remove clear protective liner from the patch
- •
- apply to affected area no more than three (3) times per day
- •
- leave in place for up to 8 but no more than 12 hours
- •
- wash hands thoroughly after applying or removing patch
- •
- some individuals may not experience pain relief until several minutes or hours after applying a patch
- •
- do not use other local anesthetic products in addition to Lidocare.
- •
- Patches may be cut into smaller sizes with scissors prior to the release of the liner. Safely discard the remaining unused pieces of cut patches where children and pets cannot get them.
- Inactive Ingredients
-
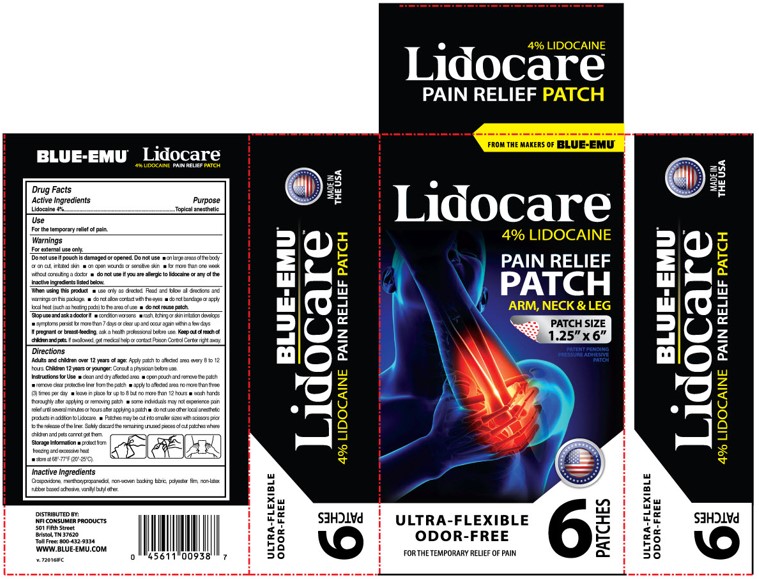
PRINCIPAL DISPLAY PANEL – 6 Patch Carton Label
FROM THE MAKERS OF BLUE-EMU®
Lidocare™
4% LIDOCAINE
PAIN RELIEF
PATCH
ARM, NECK & LEG
PATCH SIZE
1.25” x 6”
PATENT PENDING
PRESSURE ADHESIVE
PATCH
ULTRA-FLEXIBLE
ODOR-FREE
FOR THE TEMPORARY RELIEF OF PAIN
6 PATCHES
DISTRIBUTED BY:
NFI CONSUMER PRODUCTS
501 Fifth Street
Bristol, TN 37620
Toll Free: 800-432-9334
WWW.BLUE-EMU.COM -
INGREDIENTS AND APPEARANCE
LIDOCARE ARM, NECK, LEG
4% lidocaine patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69993-450 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 11 mg Inactive Ingredients Ingredient Name Strength 3-(L-MENTHOXY)-2-METHYLPROPANE-1,2-DIOL (UNII: 649M1Y2P72) VANILLYL BUTYL ETHER (UNII: S2ULN37C9R) CROSPOVIDONE (UNII: 2S7830E561) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69993-450-06 6 in 1 CARTON 05/01/2016 1 1 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 05/01/2016 Labeler - NFI, LLC (121681919) Establishment Name Address ID/FEI Business Operations ProSolus, Inc. 969793079 MANUFACTURE(69993-450)