CANKER SORE HEALING DOTS- sodium borate, calendula officinalis flowering top, causticum, daphne mezereum bark and sodium chloride tablet
Hyland's Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Canker Sore Healing Dots
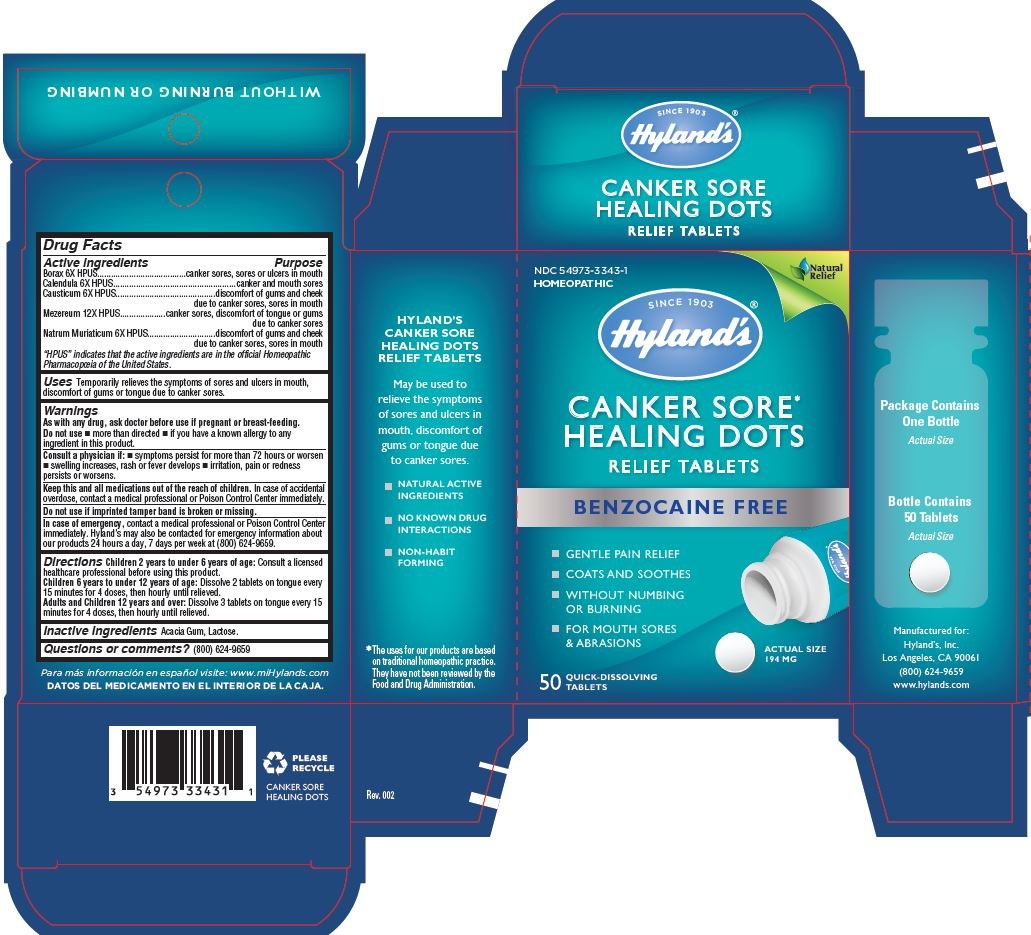
Drug Facts
|
Active ingredients |
Purpose |
|
Borax 6X HPUS |
canker sores, sores or ulcers in mouth |
|
Calendula 6X HPUS |
canker and mouth sores |
|
Causticum 6X HPUS |
discomfort of gums and cheek due to canker sores, sores in mouth |
|
Mezereum 12X HPUS |
canker sores, discomfort of tongue or gums due to canker sores |
|
Natrum Muriaticum 6X HPUS |
discomfort of gums and cheek due to canker sores, sores in mouth |
“HPUS” indicates that the active ingredients are in the official Homeopathic Pharmacopœia of the United States.
Uses
Temporarily relieves the symptoms of sores and ulcers in mouth,discomfort of gums or tongue due to canker sores.
As with any drug, ask doctor before use if pregnant or breast-feeding.
Do not use•more than directed •if you have a known allergy to any ingredient in this product.
Consult a physician
Consult a physician if:
•symptoms persist for more than 72 hours or worsen
•swelling increases, rash or fever develops
•irritation, pain or redness persists or worsens.
Directions
Children 2 years to under 6 years of age:Consult a licensed healthcare professional before using this product.
Children 6 years to under 12 years of age:Dissolve 2 tablets on tongue every 15 minutes for 4 doses, then hourly until relieved.
Adults and Children 12 years and over:Dissolve 3 tablets on tongue every 15 minutes for 4 doses, then hourly until relieved.
Temporarily relieves the symptoms of sores and ulcers in mouth, discomfort of gums or tongue due to canker sores.
PRINCIPAL DISPLAY PANEL - 50 TABLET CARTON
NDC 54973-3343-1
HOMEOPATHIC
Natural
Relief
SINCE 1903
Hyland's®
CANKER SORE*
HEALING DOTS
RELIEF TABLETS
BENZOCAINE FREE
GENTLE PAIN RELIEF
COATES AND SOOTHES
WITHOUT NUMBING
OR BURNING
FOR MOUTH SORES
& ABRASIONS
50 QUICK-DISSOLVING
TABLETS
ACTUAL SIZE
194 MG
| CANKER SORE HEALING DOTS
sodium borate, calendula officinalis flowering top, causticum, daphne mezereum bark and sodium chloride tablet |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Hyland's Inc. (008316655) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Hyland's Inc. | 008316655 | manufacture(54973-3343) , pack(54973-3343) , label(54973-3343) | |