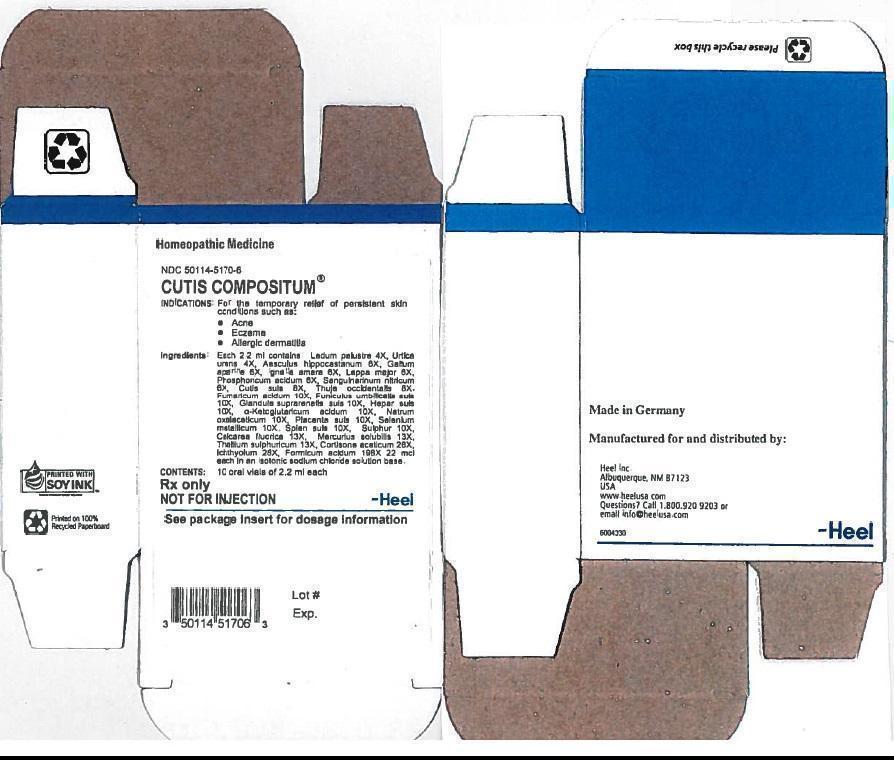
CUTIS COMPOSITUM- ledum palustre twig, urtica urens, aesculus hippocastanum flower, galium aparine, strychnos ignatii seed, arctium lappa root, phosphoric acid, sus scrofa skin, thuja occidentalis leafy twig, fumaric acid, sus scrofa umbilical cord, sus scrofa adrenal gland, pork liver, .alpha.-ketoglutaric acid, sodium diethyl oxalacetate, sus scrofa placenta, selenium, sus scrofa spleen, sulfur, calcium fluoride, mercurius solubilis, cortisone acetate, thallium sulfate, ichthammol and formic acid solution
Heel Inc
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Cutis compositum oral vial
DESCRIPTION
Each 2.2 ml ampule contains:
|
Ingredient Name |
Potency |
Quantity |
|
Aesculus hippocastanum |
6x |
22µ |
|
a-Ketoglutaricum acidum |
10x |
22µ |
|
Calcarea fluorica |
13x |
22µ |
|
Cortisone aceticum |
28x |
22µ |
|
Cutis suis |
8x |
22µ |
|
Formicum acidum |
10x |
22µ |
|
Fumaricum acidum |
198x |
22µ |
|
Funiculus umbilicals suis |
10x |
22µ |
|
Galium aparine |
6x |
22µ |
|
Glandula suprarenalis |
10x |
22µ |
|
Hepar suis |
10x |
22µ |
|
Ichthyolum |
28x |
22µ |
|
Ignatia amara |
6x |
22µ |
|
Lappa major |
6x |
22µ |
|
Ledum palustre |
4x |
22µ |
|
Mercurius solubilis |
13x |
22µ |
|
Natrum oxalaceticum |
10x |
22µ |
|
Phosphoricum acidum |
6x |
22µ |
|
Placenta suis |
10x |
22µ |
|
Selinium metallicum |
10x |
22µ |
|
Splen suis |
10x |
22µ |
|
Sulfur |
10x |
22µ |
|
Thallium sulphuricum |
13x |
22µ |
|
Thuja occidentalis |
8x |
22µ |
|
Urtica urens |
4x |
22µ |
Inactive Ingredient: Isotonic Sodium Chloride solution
INDICATION AND USAGE
Cutis compositum® Oral Vials is a homeopathic drug product is indicated for the temporary relief of persistent skin conditions such as acne, eczema, and allergic dermatitis
DOSAGE AND ADMINISTRATION
Dosage:
Adults and children above 6 years: 1 vial orally 1-3 times daily
Children up to 6 years: ½ vial orally 1-3 times daily
CONTRAINDICATIONS
Cutis compositum®Oral Vials are contraindicated in patients with known hypersensitivity to Cutis compositum® or any of its ingredients
ADVERSE REACTIONS
No adverse events have been reported with a causal relationship to Cutis compositum® Oral Vials
OVERDOSAGE
Overdosage: No negative effects of an overdose have been reported and none are expected due to the homeopathic dilutions
| CUTIS COMPOSITUM
ledum palustre twig, urtica urens, aesculus hippocastanum flower, galium aparine, strychnos ignatii seed, arctium lappa root, phosphoric acid, sus scrofa skin, thuja occidentalis leafy twig, fumaric acid,sus scrofa umbilical cord,sus scrofa adrenal gland,pork liver,.alpha.-ketoglutaric acid, sodium diethyl oxalacetate,sus scrofa placenta,selenium,sus scrofa spleen,sulfur,calcium fluoride, mercurius solubilis,thallium sulfate,cortisone acetate, ichthammol, formic acid, solution |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Heel Inc (102783016) |