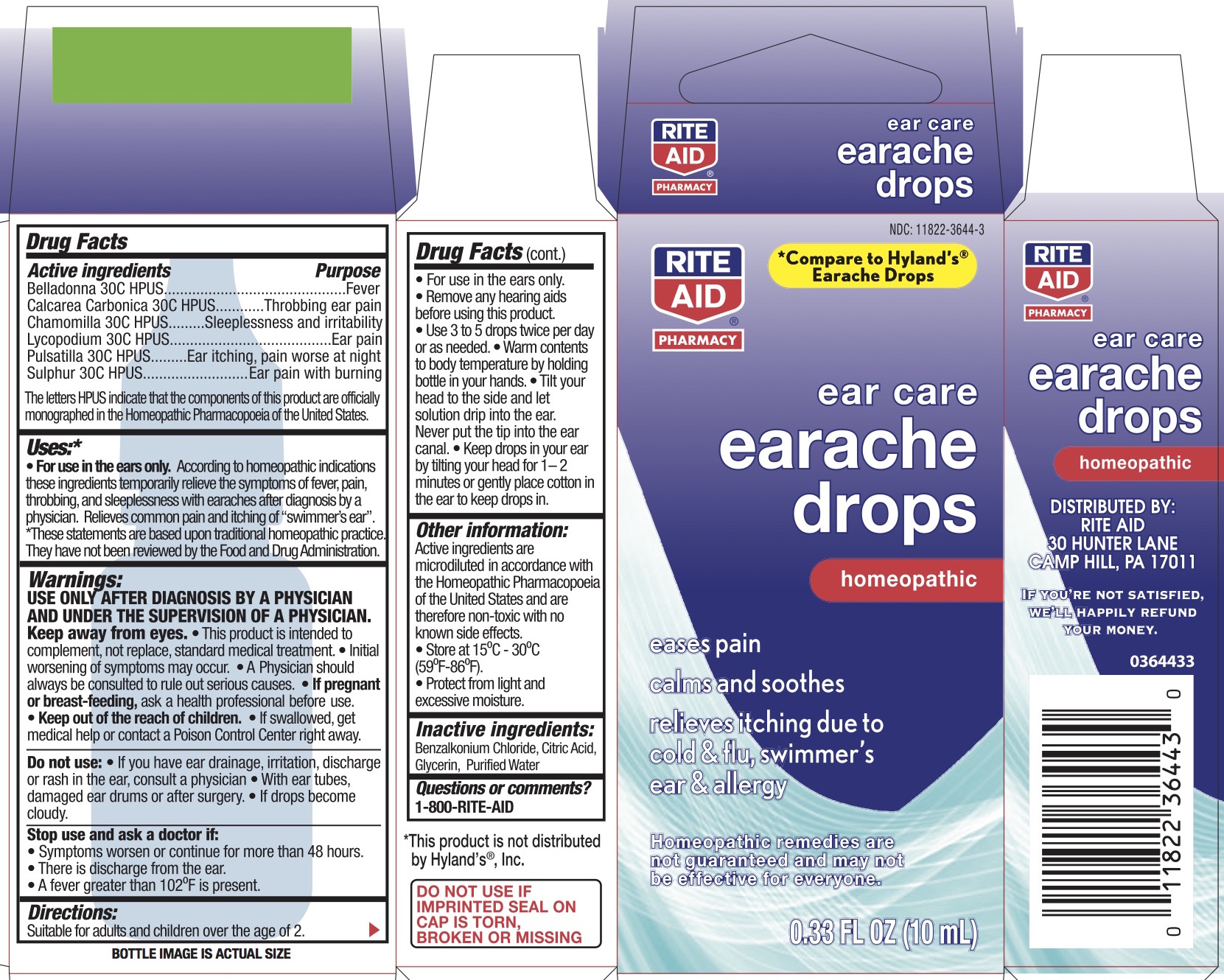
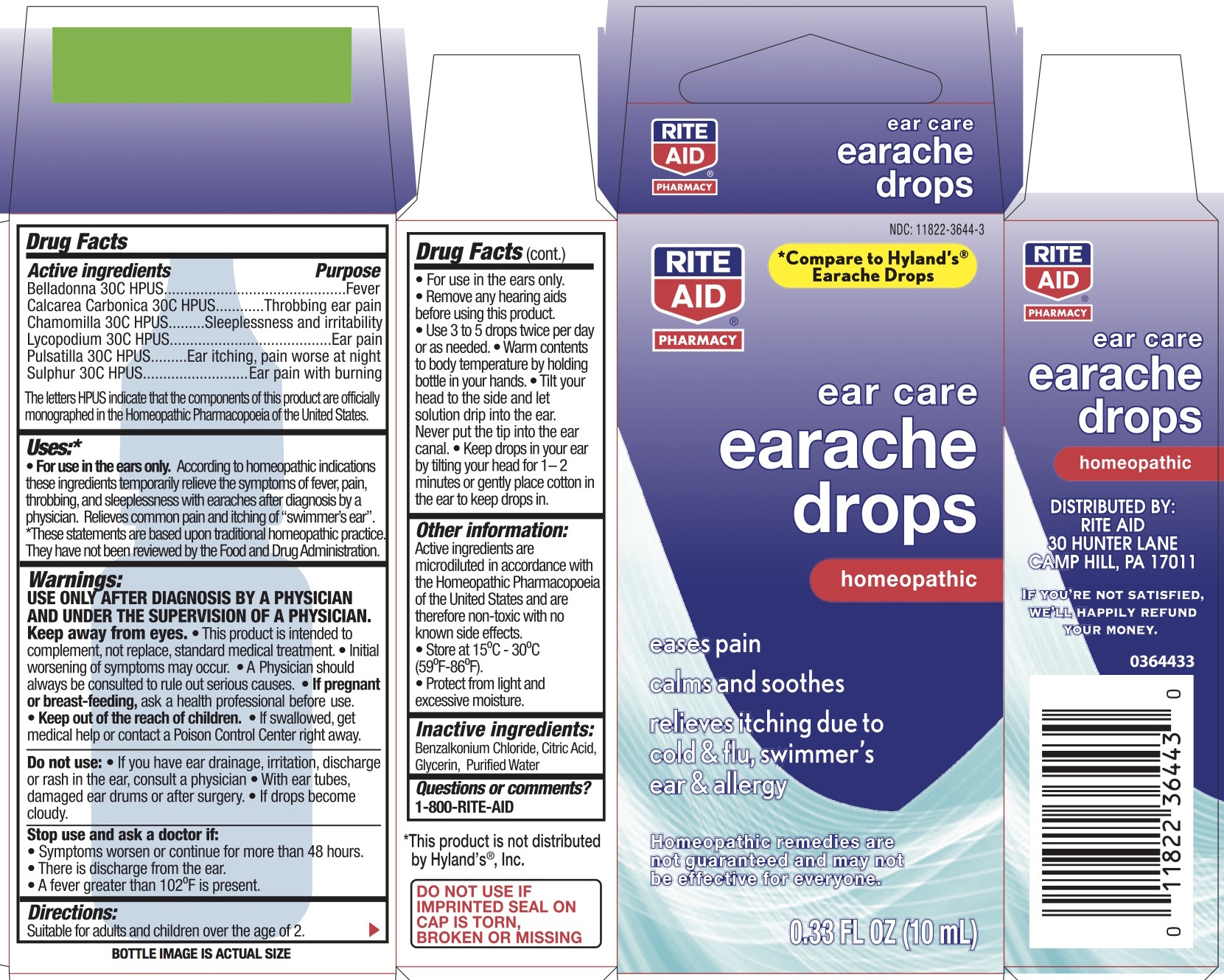
Label: EAR CARE EARACHE DROPS- atropa belladonna, anemone pulsatilla, lycopodium clavatum spore, matricaria chamomilla, and sulfur liquid
- NDC Code(s): 11822-3644-3
- Packager: Rite Aid Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated March 16, 2018
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
ACTIVE INGREDIENT
Active ingredients Purpose "HPUS" indicates the components of this product are officially monographed in the Homeopathic Pharmacopœia of the United States. Belladonna 30C HPUS Fever Calcarea Carbonica 30C HPUS Throbbing ear pain Chamomilla 30C HPUS Sleeplessness and irritability Lycopodium 30C HPUS Ear pain Pulsatilla 30C HPUS Ear itching, pain worse at night Sulphur 30C HPUS Ear pain with burning -
Uses*
• For use in the ears only. According to homeopathic indications these ingredients temporarily relieve the symptoms of fever, pain, throbbing, and sleeplessness with earaches after diagnosis by a physician. Relieves common pain and itching of “swimmers ear”. *These statements are based upon traditional homeopathic practice. They have not been reviewed by the Food and Drug Administration.
-
Warnings
USE ONLY AFTER DIAGNOSIS BY A PHYSICIAN AND UNDER THE SUPERVISION OF A PHYSICIAN. Keep away from eyes. This product is intended to complement, not replace, standard medical treatment. Initial worsening of symptoms may occur. A physician should always be consulted to rule out serious causes.
Stop and ask a doctor it:
- Symptoms worsen or continue for more than 48 hours
- There is discharge from the ear.
- A fever of greater than 102° is present
-
Directions
Suitable for adults and children over the age of 2.
• For use in the ears only
• Remove any hearing aids
• Use 3-5 drops twice per day or as needed. Warm contents to body temperature by holding bottle in your hands. Tilt ear upward for at least 2 minutes after application or gently place cotton in ear to keep drops in. Tilt your head to the side and let solution drip into the ear. Never put the tip in the ear canal. Keep drops in your ear by tilting your head for 1-2 minutes or gently place cotton in the ear to keep drops in.
- Inactive ingredients
- Questions or comments?
- Other Information:
- PRINCIPAL DISPLAY PANEL - 10 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
EAR CARE EARACHE DROPS
atropa belladonna, anemone pulsatilla, lycopodium clavatum spore, matricaria chamomilla, and sulfur liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11822-3644 Route of Administration AURICULAR (OTIC) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ATROPA BELLADONNA (UNII: WQZ3G9PF0H) (ATROPA BELLADONNA - UNII:WQZ3G9PF0H) ATROPA BELLADONNA 30 [hp_C] in 1 mL OYSTER SHELL CALCIUM CARBONATE, CRUDE (UNII: 2E32821G6I) (OYSTER SHELL CALCIUM CARBONATE, CRUDE - UNII:2E32821G6I) OYSTER SHELL CALCIUM CARBONATE, CRUDE 30 [hp_C] in 1 mL MATRICARIA CHAMOMILLA (UNII: G0R4UBI2ZZ) (MATRICARIA CHAMOMILLA - UNII:G0R4UBI2ZZ) MATRICARIA CHAMOMILLA 30 [hp_C] in 1 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 30 [hp_C] in 1 mL ANEMONE PULSATILLA (UNII: I76KB35JEV) (ANEMONE PULSATILLA - UNII:I76KB35JEV) ANEMONE PULSATILLA 30 [hp_C] in 1 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 30 [hp_C] in 1 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) WATER (UNII: 059QF0KO0R) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11822-3644-3 1 in 1 CARTON 08/03/2017 1 10 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 08/03/2017 Labeler - Rite Aid Corporation (014578892)