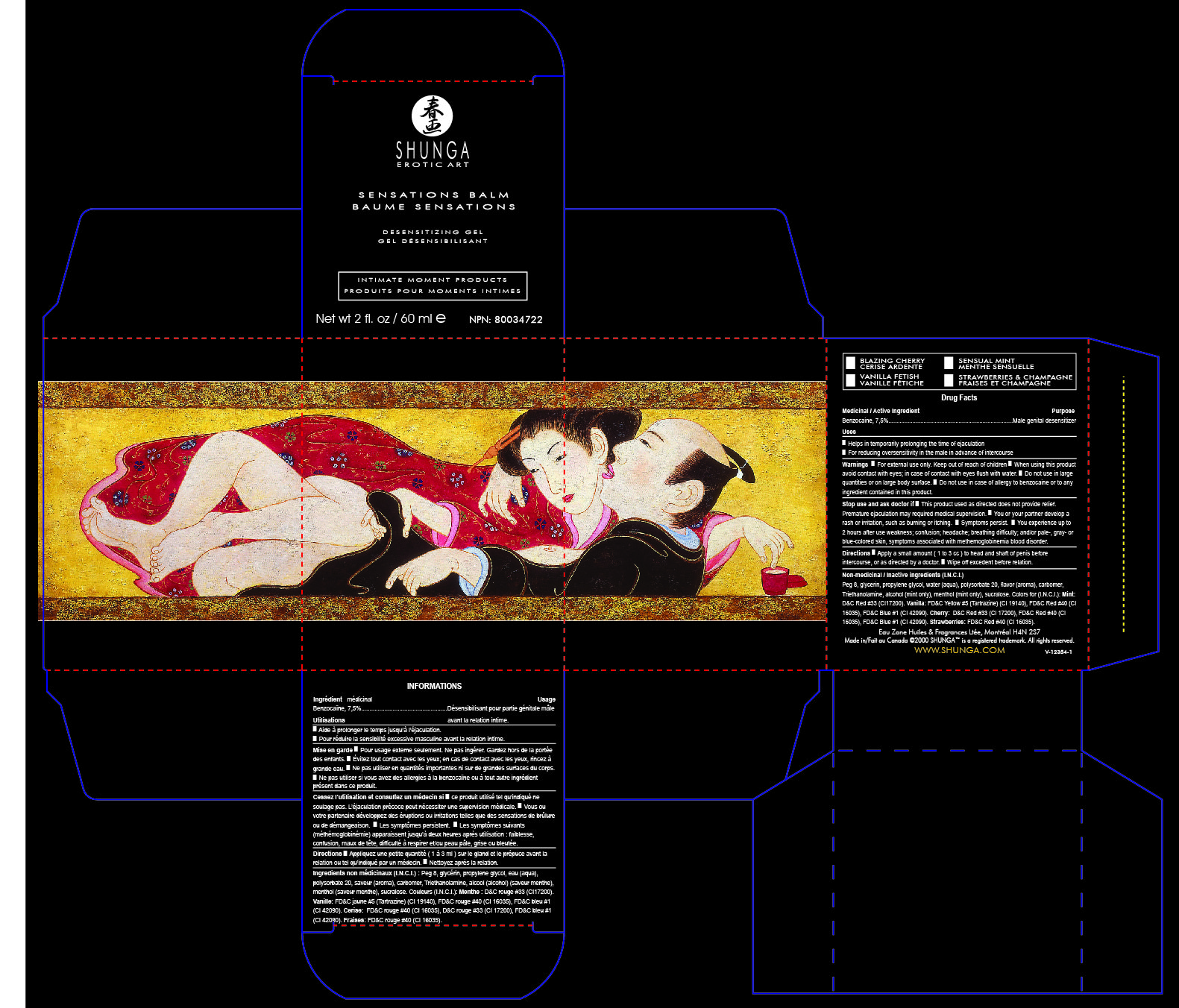
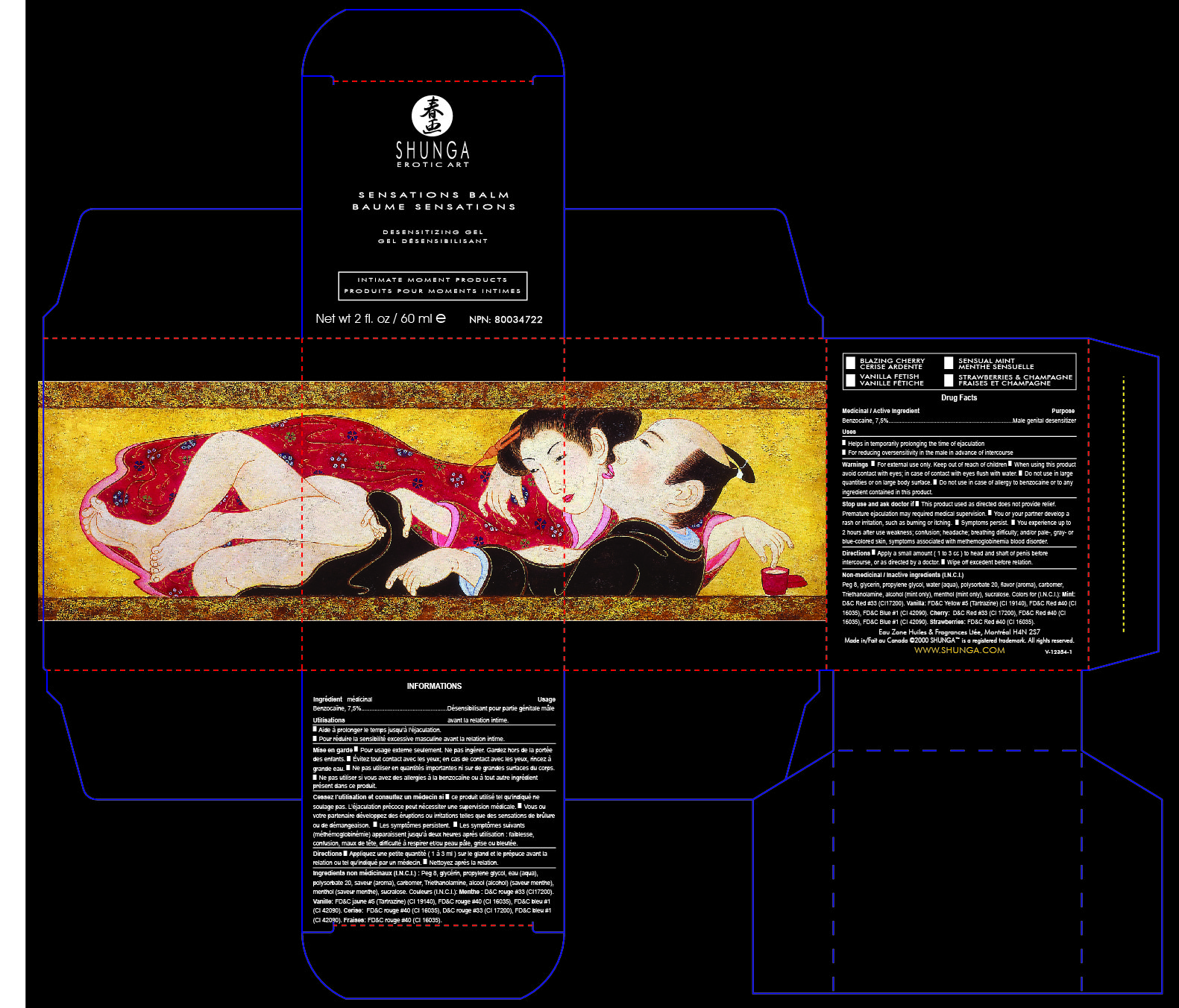
Label: SHUNGA SENSATION BALM- benzocain gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 63240-5000-1, 63240-5000-2, 63240-5001-1, 63240-5001-2, view more63240-5002-1, 63240-5002-2, 63240-5003-1, 63240-5003-2 - Packager: Eau Zone Oils and Fragrances LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 9, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
-
Warnings
■ For external use only. Keep out of reach of children ■ When using this product
avoid contact with eyes; in case of contact with eyes flush with water. ■ Do not use in large
quantities or on large body surface. ■ Do not use in case of allergy to benzocaine or to any
ingredient contained in this product. - Directions
-
Stop use and ask doctor if
Stop use and ask doctor if ■ This product used as directed does not provide relief.
Premature ejaculation may required medical supervision. ■ You or your partner develop a
rash or irritation, such as burning or itching. ■ Symptoms persist. ■ You experience up to
2 hours after use weakness; confusion; headache; breathing difficulty; and/or pale-, gray- or
blue-colored skin, symptoms associated with methemoglobinemia blood disorder. -
Warning
Warnings
For external use only. Keep out of reach of children
When using this product avoid contact with eyes; in case of contact with eyes flush with water.
Do not use in large quantities or on large body surface.
Do not use in case of allergy to benzocaine or to any ingredient contained in this product.
-
Purpose
Name: SHUNGA Sensation Balm
SHUNGA Male Genital Desensitizer helps in temporarily prolonging the time until ejaculation by diminishing tactile sensations to the penis.
Purpose
Male genital desensitizer
Uses
Helps in temporarily prolonging the time of ejaculation ■ For reducing oversensitivity in the male in advance of intercourseDirections ■ Apply a small amount ( 1 to 3 cc ) to head and shaft of penis before
intercourse, or as directed by a doctor. ■ Wipe off excedent before relation.
-
Non-medicinal / Inactive ingredients (I.N.C.I.)
Non-medicinal / Inactive ingredients (I.N.C.I.)
Water (aqua), peg 8, glycerin, propylene glycol, triethanolamine, carbomer, flavor (aroma),
sucralose. Colors for (I.N.C.I.): Mint : D&C Red #33 (CI17200). Vanilla: FD&C Yellow #5
(Tartrazine) (CI 19140), FD&C Red #40 (CI 16035), FD&C Blue #1 (CI 42090). Cherry and
Strawberries: FD&C Red #40 (CI 16035), D&C Red #33 (CI 17200).Eau Zone Huiles & Fragrances Ltée, Montréal H4N 2S7
Made in/Fait au Canada ©2000 SHUNGA™ is a registered trademark. All rights reserved. - Directions
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SHUNGA SENSATION BALM
benzocain gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63240-5000 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 5.07 g in 67.5 g Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) 18 g in 67.5 g GLYCERIN (UNII: PDC6A3C0OX) 20.86 g in 67.5 g PROPYLENE GLYCOL (UNII: 6DC9Q167V3) 15 g in 67.5 g WATER (UNII: 059QF0KO0R) 5.4 g in 67.5 g POLYSORBATE 20 (UNII: 7T1F30V5YH) 0.66 g in 67.5 g CARBOMER 940 (UNII: 4Q93RCW27E) 0.357 g in 67.5 g TROLAMINE (UNII: 9O3K93S3TK) 0.287 g in 67.5 g SUCRALOSE (UNII: 96K6UQ3ZD4) 0.006 g in 67.5 g D&C RED NO. 33 (UNII: 9DBA0SBB0L) 0.0027 g in 67.5 g FD&C RED NO. 40 (UNII: WZB9127XOA) 0.0003 g in 67.5 g FD&C BLUE NO. 1 (UNII: H3R47K3TBD) 0.0001 g in 67.5 g Product Characteristics Color red Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63240-5000-2 1 in 1 BOX 01/01/2000 1 NDC:63240-5000-1 67.5 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part348 01/01/2000 SHUNGA SENSATION BALM
benzocain gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63240-5001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 5.06 g in 67.5 g Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) 18 g in 67.5 g GLYCERIN (UNII: PDC6A3C0OX) 20.41 g in 67.5 g PROPYLENE GLYCOL (UNII: 6DC9Q167V3) 15 g in 67.5 g WATER (UNII: 059QF0KO0R) 5.4 mL in 67.5 g POLYSORBATE 20 (UNII: 7T1F30V5YH) 0.66 g in 67.5 g CARBOMER 940 (UNII: 4Q93RCW27E) 0.357 g in 67.5 g TROLAMINE (UNII: 9O3K93S3TK) 0.287 g in 67.5 g ALCOHOL (UNII: 3K9958V90M) 0.09 mL in 67.5 g MENTHOL (UNII: L7T10EIP3A) 0.024 g in 67.5 g SUCRALOSE (UNII: 96K6UQ3ZD4) 0.006 g in 67.5 g D&C RED NO. 33 (UNII: 9DBA0SBB0L) 0.0027 g in 67.5 g Product Characteristics Color red Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63240-5001-2 1 in 1 BOX 01/01/2000 1 NDC:63240-5001-1 67.5 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part348 01/01/2000 SHUNGA SENSATION BALM
benzocain gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63240-5002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 5.07 g in 67.5 g Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) 18 g in 67.5 g GLYCERIN (UNII: PDC6A3C0OX) 20.27 g in 67.5 g PROPYLENE GLYCOL (UNII: 6DC9Q167V3) 15 g in 67.5 g WATER (UNII: 059QF0KO0R) 5.4 mL in 67.5 g POLYSORBATE 20 (UNII: 7T1F30V5YH) 0.66 g in 67.5 g CARBOMER 940 (UNII: 4Q93RCW27E) 0.357 g in 67.5 g TROLAMINE (UNII: 9O3K93S3TK) 0.287 g in 67.5 g SUCRALOSE (UNII: 96K6UQ3ZD4) 0.006 g in 67.5 g FD&C YELLOW NO. 5 (UNII: I753WB2F1M) 0.000252 g in 67.5 g FD&C RED NO. 40 (UNII: WZB9127XOA) 0.00003 g in 67.5 g FD&C BLUE NO. 1 (UNII: H3R47K3TBD) 0.000006 g in 67.5 g Product Characteristics Color white Score Shape Size Flavor BUTTERSCOTCH Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63240-5002-2 1 in 1 BOX 01/01/2000 1 NDC:63240-5002-1 67.5 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part348 01/01/2000 SHUNGA SENSATION BALM
benzocain gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63240-5003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 5.07 g in 67.5 g Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) 18 g in 67.5 g GLYCERIN (UNII: PDC6A3C0OX) 20.86 g in 67.5 g PROPYLENE GLYCOL (UNII: 6DC9Q167V3) 15 g in 67.5 g WATER (UNII: 059QF0KO0R) 5.4 mL in 67.5 g POLYSORBATE 20 (UNII: 7T1F30V5YH) 0.66 g in 67.5 g CARBOMER 940 (UNII: 4Q93RCW27E) 0.357 g in 67.5 g TROLAMINE (UNII: 9O3K93S3TK) 0.287 g in 67.5 g SUCRALOSE (UNII: 96K6UQ3ZD4) 0.006 g in 67.5 g FD&C RED NO. 40 (UNII: WZB9127XOA) 0.00054 g in 67.5 g Product Characteristics Color pink Score Shape Size Flavor STRAWBERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63240-5003-2 1 in 1 BOX 01/01/2005 1 NDC:63240-5003-1 67.5 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part348 01/01/2005 Labeler - Eau Zone Oils and Fragrances LTD (249543091) Establishment Name Address ID/FEI Business Operations Eau Zone Oils and Fragrances LTD 249543091 manufacture(63240-5000) , label(63240-5000, 63240-5001, 63240-5002, 63240-5003)