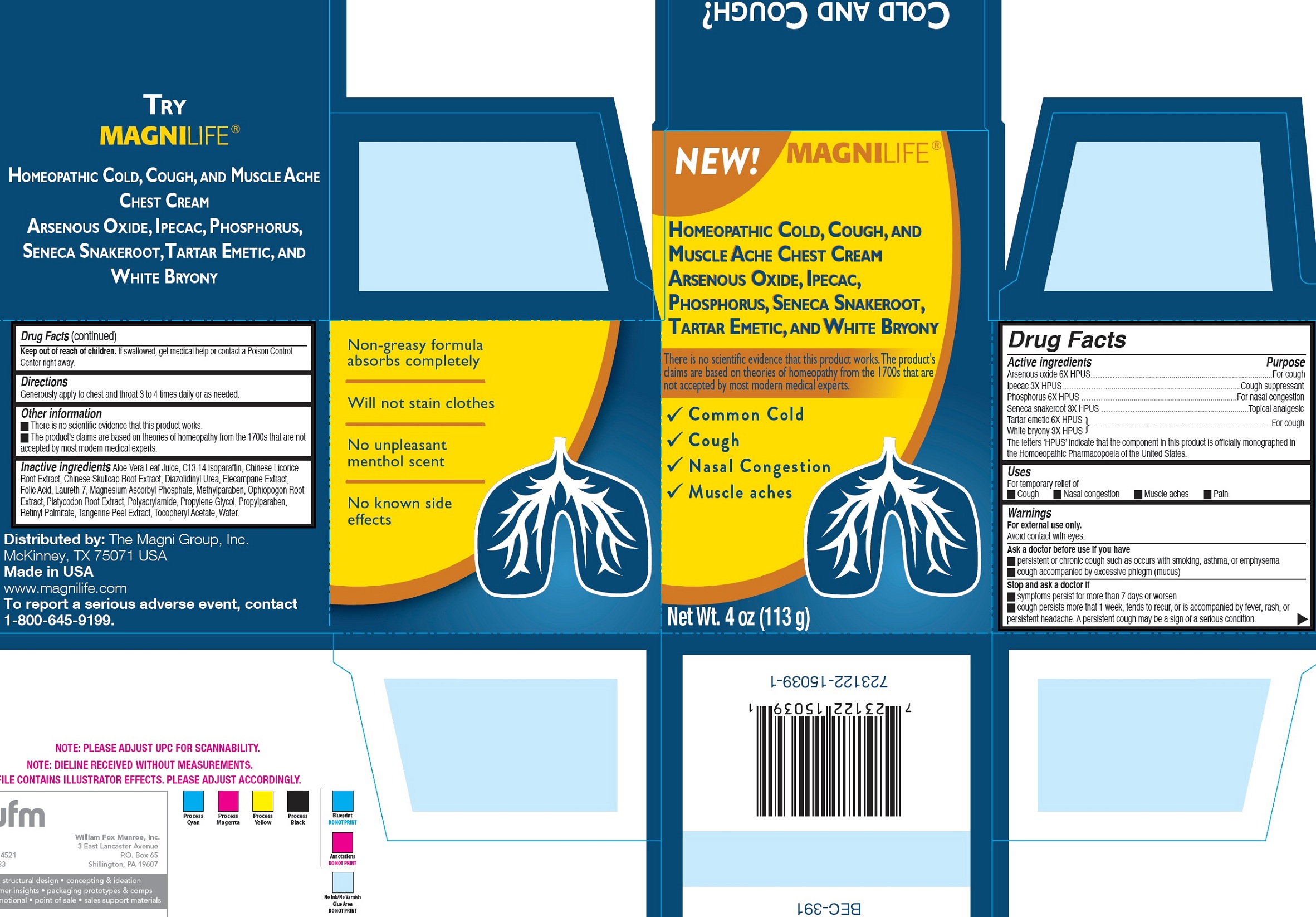
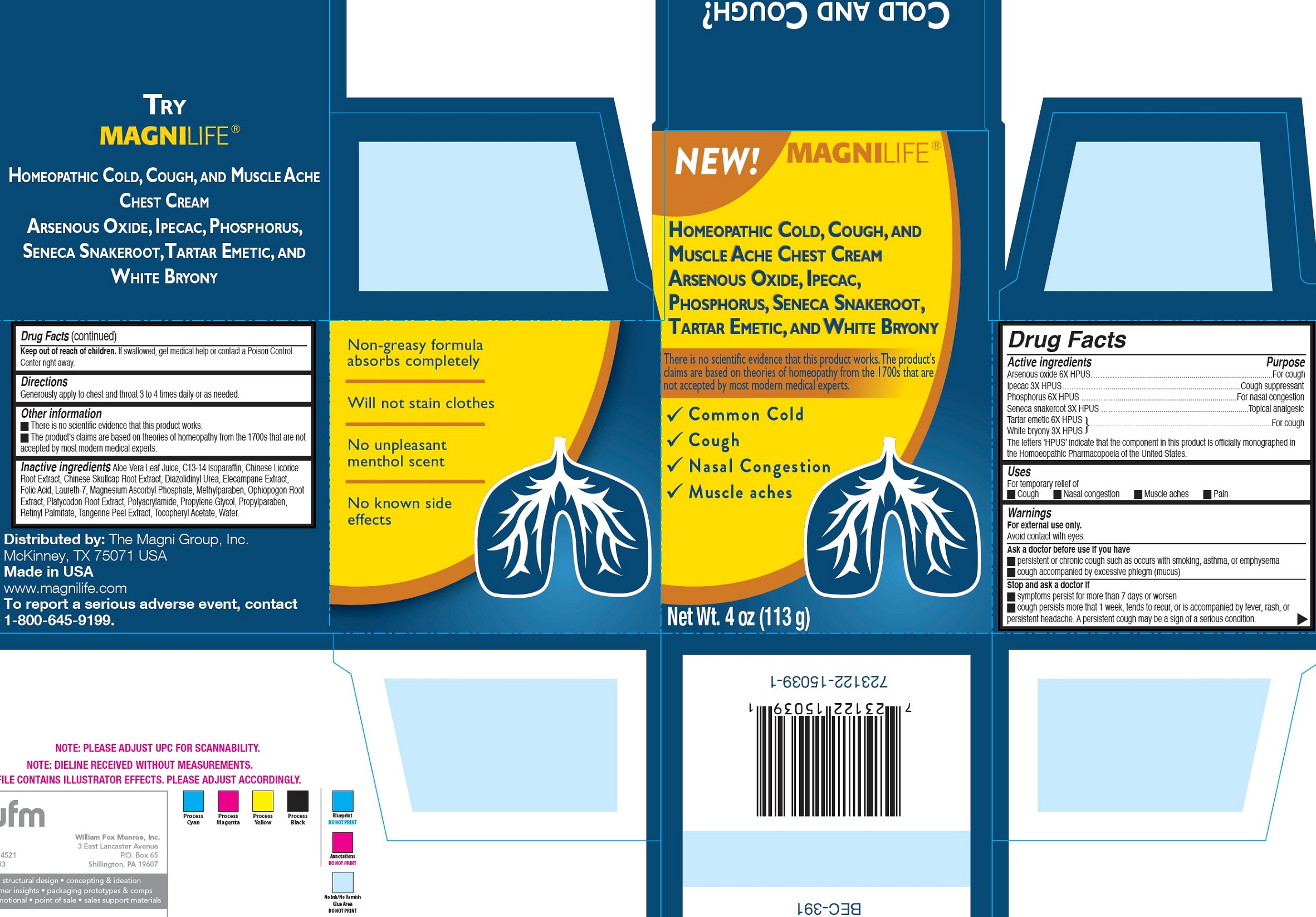
Label: BREATHE EASY CHEST- arsenic trioxide, ipecac, phosphorus, polygala senega root, antimony potassium tartrate, bryonia alba whole cream
- NDC Code(s): 43689-0032-1
- Packager: Magni Group
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated November 15, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Uses
-
Warnings
For external use only.
Avoid contact with eyes.Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, or emphysema
- cough accompanied by excessive phlegm (mucus)
- Directions
- Other information
-
Inactive ingredients
Aloe Vera Leaf Juice, C13-14 Isoparaffin, Chinese Licorice Root Extract, Chinese Skullcap Root Extract, Diazolidinyl Urea, Elecampane Extract, Folic Acid, Laureth-7, Magnesium Ascorbyl Phosphate, Methylparaben, Ophiopogon Root Extract, Platycodon Root Extract, Polyacrylamide, Propylene Glycol, Propylparaben, Retinyl Palmitate, Tangerine Peel Extract, Tocopheryl Acetate, Water.
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
BREATHE EASY CHEST
arsenic trioxide, ipecac, phosphorus, polygala senega root, antimony potassium tartrate, bryonia alba whole creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43689-0032 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 6 [hp_X] in 113 g IPECAC (UNII: 62I3C8233L) (IPECAC - UNII:62I3C8233L) IPECAC 3 [hp_X] in 113 g PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 6 [hp_X] in 113 g POLYGALA SENEGA ROOT (UNII: M7T6H7D4IF) (POLYGALA SENEGA ROOT - UNII:M7T6H7D4IF) POLYGALA SENEGA ROOT 3 [hp_X] in 113 g ANTIMONY POTASSIUM TARTRATE (UNII: DL6OZ476V3) (ANTIMONY CATION (3+) - UNII:069647RPT5) ANTIMONY POTASSIUM TARTRATE 6 [hp_X] in 113 g BRYONIA ALBA WHOLE (UNII: 56K0VVT47P) (BRYONIA ALBA WHOLE - UNII:56K0VVT47P) BRYONIA ALBA WHOLE 3 [hp_X] in 113 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) INULA HELENIUM ROOT (UNII: E55SMD6DA8) FOLIC ACID (UNII: 935E97BOY8) LAURETH-7 (UNII: Z95S6G8201) MAGNESIUM ASCORBYL PHOSPHATE (UNII: 0R822556M5) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43689-0032-1 1 in 1 CARTON 07/16/2017 1 113 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 07/16/2017 Labeler - Magni Group (113501902)