INTRALIPID - soybean oil, egg phospholipids and glycerin emulsion
Baxter Healthcare CORP
----------
Intralipid® 10%
A 10% I.V. Fat Emulsion In Excel® Container
Deaths in preterm infants after infusion of intravenous fat emulsion have been reported in the medical literature.2 Autopsy findings included intravascular fat accumulation in the lungs. Treatment of premature and low birth weight infants with intravenous fat emulsion must be based upon careful benefit-risk assessment. Strict adherence to the recommended total daily dose is mandatory; hourly infusion rate should be as slow as possible in each case and fat should not in any case exceed 1 g fat/kg in four hours. Premature and small for gestational age infants have poor clearance of intravenous fat emulsion and increased free fatty acid plasma levels following fat emulsion infusion; therefore, serious consideration must be given to administration of less than the maximum recommended doses in these patients in order to decrease the likelihood of intravenous fat overload. The infant’s ability to eliminate the infused fat from the circulation must be carefully monitored (such as serum triglycerides and/or plasma free fatty acid levels). The lipemia must clear between daily infusions.
DESCRIPTION
Intralipid® 10% (A 10% Intravenous Fat Emulsion) is a sterile, non-pyrogenic fat emulsion prepared for intravenous administration as a source of calories and essential fatty acids. It is made up of 10% Soybean Oil, 1.2% Egg Yolk Phospholipids, 2.25% Glycerin, and Water for Injection. In addition, sodium hydroxide has been added to adjust the pH so that the final product pH is 8. pH range is 6 to 8.9.
The soybean oil is a refined natural product consisting of a mixture of neutral triglycerides of predominantly unsaturated fatty acids with the following structure:
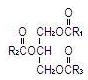
where 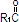 ,
, 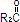 and
and  are saturated and unsaturated fatty acid residues.
are saturated and unsaturated fatty acid residues.
The major component fatty acids are linoleic (44-62%), oleic (19-30%), palmitic (7-14%), linolenic (4-11%) and stearic (1.4-5.5%)1. These fatty acids have the following chemical and structural formulas:
| Linoleic acid C18H32O2 | 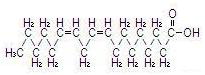 |
| Oleic acid C18H34O2 | 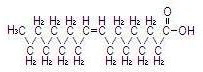 |
| Palmitic acid C16H32O2 | 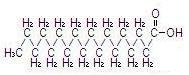 |
| Linolenic acid C18H30O2 | 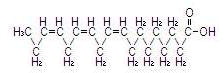 |
| Stearic acid C18H36O2 | 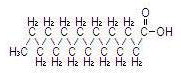 |
Purified egg phosphatides are a mixture of naturally occurring phospholipids which are isolated from the egg yolk. These phospholipids have the following general structure:
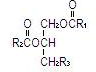
 and
and 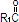 contain saturated and unsaturated fatty acids that abound in neutral fats. R3 is primarily either the choline or ethanolamine ester of phosphoric acid.
contain saturated and unsaturated fatty acids that abound in neutral fats. R3 is primarily either the choline or ethanolamine ester of phosphoric acid.
| Phosphatidylcholine | Phosphatidylethanolamine |
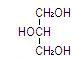
Glycerin is chemically designated C3H8O3 and is a clear colorless, hygroscopic syrupy liquid. It has the following structural formula:
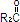
Intralipid® 10% (A 10% Intravenous Fat Emulsion) has an osmolality of approximately 300 mOsmol/kg water (which represents 260 mOsmol/liter of emulsion) and contains emulsified fat particles of approximately 0.5 micron size.
The total caloric value, including fat, phospholipid and glycerin, is 1.1 kcal per mL of Intralipid 10%. The phospholipids present contribute 47 milligrams or approximately 1.5 mmol of phosphorus per 100 mL, of the emulsion.
The primary container is manufactured from Excel® film, a polypropylene based material comprised of three co-extruded layers.
The plastic container is made from multilayered film specifically designed for parenteral drugs. It contains no plasticizers and exhibits virtually no leachables. The solution contact layer is a rubberized copolymer of ethylene and propylene. The container is nontoxic and biologically inert. The container-solution unit is a closed system and is not dependent upon entry of external air during administration. The container is overwrapped to provide protection from the physical environment and to provide an additional moisture barrier when necessary.
CLINICAL PHARMACOLOGY
Intralipid® 10% is metabolized and utilized as a source of energy causing an increase in heat production, decrease in respiratory quotient and increase in oxygen consumption. The infused fat particles are cleared from the blood stream in a manner thought to be comparable to the clearing of chylomicrons.
Intralipid® 10% will prevent the biochemical lesions of essential fatty acid deficiency (EFAD), and correct the clinical manifestations of the EFAD syndrome.
INDICATIONS AND USAGE
Intralipid® 10% is indicated as a source of calories and essential fatty acids for patients requiring parenteral nutrition for extended periods of time (usually for more than 5 days) and as a source of essential fatty acids for prevention of EFAD.
CONTRAINDICATIONS
The administration of Intralipid® 10% is contraindicated in patients with disturbances of normal fat metabolism such as pathologic hyperlipemia, lipoid nephrosis or acute pancreatitis if accompanied by hyperlipidemia.
WARNINGS
Caution should be exercised in administering Intralipid® 10% (A 10% Intravenous Fat Emulsion) to patients with severe liver damage, pulmonary disease, anemia or blood coagulation disorders, or when there is danger of fat embolism.
WARNING: TThis product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
PRECAUTIONS
When Intralipid® 10% is administered, the patients capacity to eliminate the infused fat from the circulation must be monitored by use of an appropriate laboratory determination of serum triglycerides. Overdosage must be avoided.
During long term intravenous nutrition with Intralipid® 10%, liver function tests should be performed. If these tests indicate that liver function is impaired, the therapy should be withdrawn.
Frequent (some advise daily) platelet counts should be done in neonatal patients receiving parenteral nutrition with Intralipid® 10%.
Drug product contains no more than 25 mcg/L of aluminum.
Carcinogenesis, Mutagenesis, Impairment of Fertility: Studies with Intralipid® have not been performed to evaluate carcinogenic potential, mutagenic potential, or effects on fertility.
Pregnancy Category C: Animal reproduction studies have not been conducted with Intralipid®. It is also not known whether Intralipid® can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Intralipid® should be given to a pregnant woman only if clearly needed.
Nursing Mothers: Caution should be exercised when Intralipid® is administered to a nursing woman.
Pediatric Use: See DOSAGE AND ADMINISTRATION.
AVOID OVERDOSAGE ABSOLUTELY.
ADVERSE REACTIONS
The adverse reactions observed can be separated into two classes:
- Those more frequently encountered are due: either to contamination of the intravenous catheter and result in sepsis, or to vein irritation by concurrently infused hypertonic solutions and may result in thrombophlebitis. These adverse reactions are inseparable from the hyper-alimentation procedure with or without Intralipid® 10% (A 10% I.V. Fat Emulsion).
- .Less frequent reactions more directly related to Intralipid® 10% are: a) immediate or early adverse reactions, each of which has been reported to occur in clinical trials, in an incidence of less than 1 %; dyspnea, cyanosis, allergic reactions, hyperlipemia, hypercoagulability, nausea, vomiting, headache, flush-ing, increase in temperature, sweating, sleepiness, pain in the chest and back, slight pressure over the eyes, dizziness, and irritation at the site of infusion, and, rarely, thrombocytopenia in neonates; b) delayed adverse reactions such as hepatomegaly, jaundice due to central lobular cholestasis, splenomegaly, thrombocytopenia, leukopenia, transient increases in liver function tests, and overloading syndrome (focal seizures, fever, leukocytosis, hepatomegaly, splenomegaly and shock).
The deposition of a brown pigmentation in the reticuloendothelial system, the so-called ” intravenous fat pigment,” has been reported in patients infused with Intralipid® 10%. The causes and significance of this phenomenon are unknown.
OVERDOSAGE
In the event of fat overload during therapy, stop the infusion of Intralipid® 10% until visual inspection of the plasma, determination of triglyceride concentrations, or measurement of plasma light-scattering activity by nephelometry indicates the lipid has cleared. Re-evaluate the patient and institute appropriate corrective measures. See WARNINGS and PRECAUTIONS.
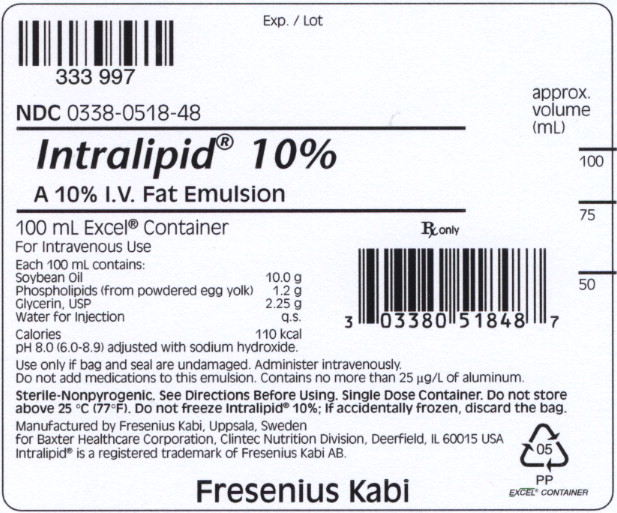
Principal Display Panel - Bag Label
NDC 0338-0518-48
Intralipid® 10%
A 10% I.V. Fat Emulsion
100 mL Excel® Container Rx only
For Intravenous Use
Each 100 mL contains:
Soybean Oil 10.0 g
Phospholipids (from powdered egg yolk) 1.2 g
Glycerin, USP 2.25 g
Water for Injection q.s.
Calories 110 kcal
pH 8.0 (6.0-8.9) adjusted with sodium hydroxide.
Use only if bag and seal are undamaged. Administer intravenously.
Do not add medications to this emulsion. Contains no more than 25 μg/L of aluminum.
Sterile-Nonpyrogenic. See Directions Before Using. Single Dose Container. Do not store
above 25°C (77°F). Do not freeze Intralipid®
10%; If accidentally frozen, discard the bag.
Manufactured by Fresenius kabi, Uppsala, Sweden
for Baxter Healthcare Corporation, Clintec Nutrition Division, Deerfield, IL 60015 USA
Intralipid® is a registered trademark of Fresenius kabi AB.
| INTRALIPID
soybean oil emulsion |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Baxter Healthcare CORP (005083209) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Fresenius Kabi AB Uppsala | 559785113 | ANALYSIS(0338-0518) , MANUFACTURE(0338-0518) | |