Label: NEUTROGENA OIL FREE ACNE WASH- salicylic acid lotion
- NDC Code(s): 69968-0516-6, 69968-0516-9
- Packager: Johnson & Johnson Consumer Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 12, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
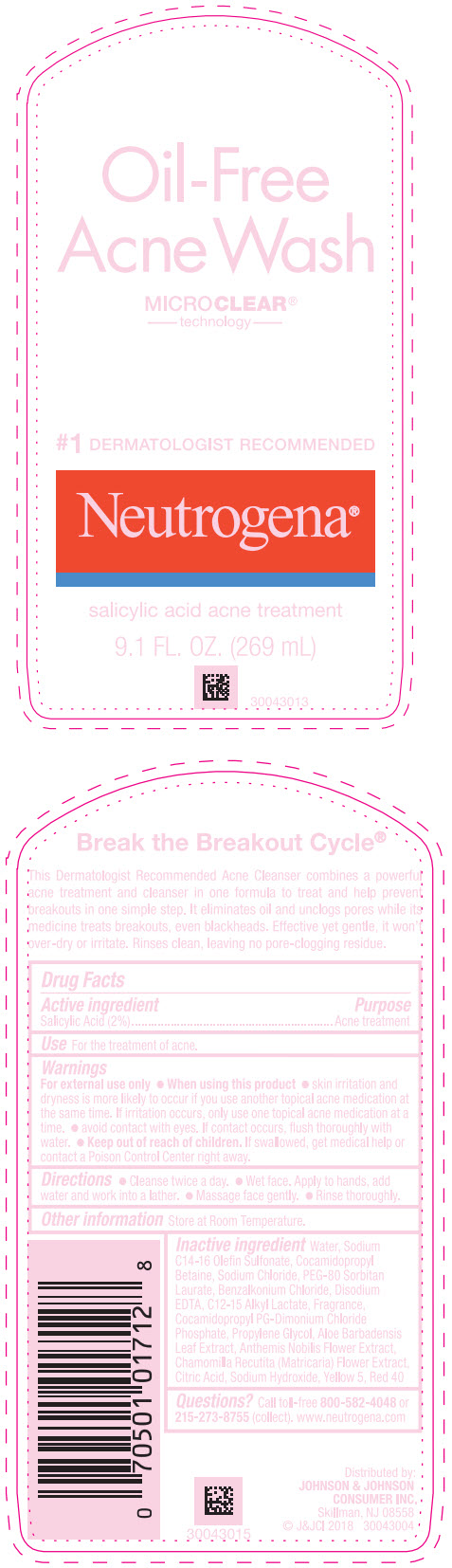
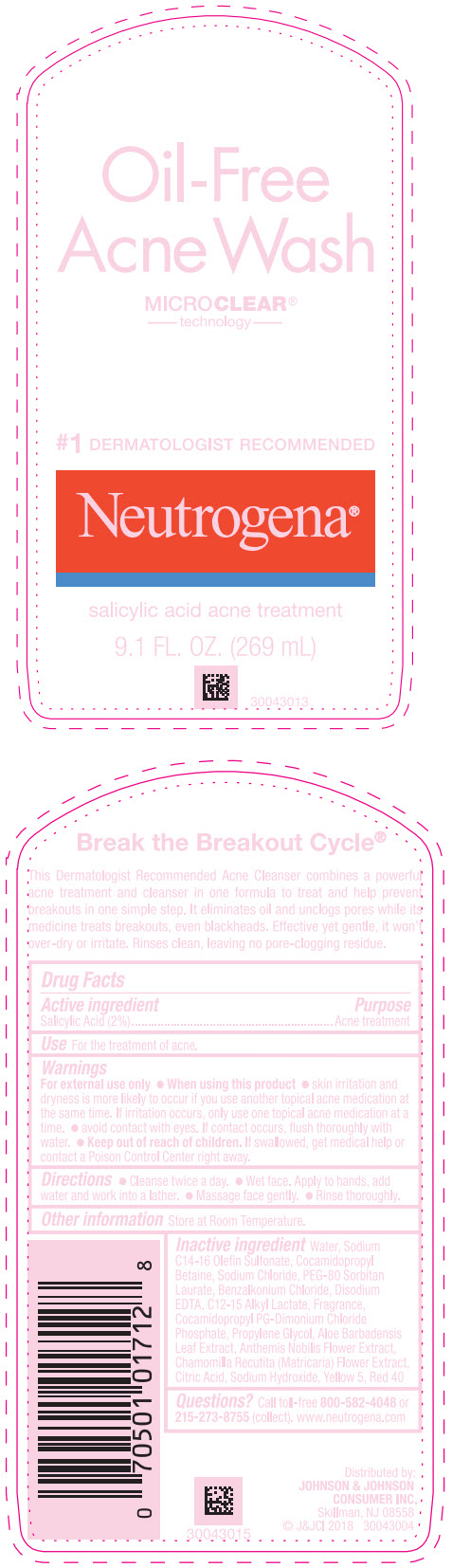
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only.
- Directions
- Other information
-
Inactive ingredients
Water, Sodium C14-16 Olefin Sulfonate, Cocamidopropyl Betaine, Sodium Chloride, PEG-80 Sorbitan Laurate, Benzalkonium Chloride, Disodium EDTA, C12-15 Alkyl Lactate, Fragrance, Cocamidopropyl PG-Dimonium Chloride Phosphate, Propylene Glycol, Aloe Barbadensis Leaf Extract, Anthemis Nobilis Flower Extract, Chamomilla Recutita (Matricaria) Flower Extract, Citric Acid, Sodium Hydroxide, Yellow 5, Red 40
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 269 mL Bottle Label
-
INGREDIENTS AND APPEARANCE
NEUTROGENA OIL FREE ACNE WASH
salicylic acid lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0516 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM C14-16 OLEFIN SULFONATE (UNII: O9W3D3YF5U) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) SODIUM CHLORIDE (UNII: 451W47IQ8X) PEG-80 SORBITAN LAURATE (UNII: 239B50Y732) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) C12-15 ALKYL LACTATE (UNII: GC844VRD7E) COCAMIDOPROPYL PROPYLENE GLYCOL-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALOE VERA LEAF (UNII: ZY81Z83H0X) MATRICARIA CHAMOMILLA WHOLE (UNII: G0R4UBI2ZZ) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) SODIUM HYDROXIDE (UNII: 55X04QC32I) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) FD&C RED NO. 40 (UNII: WZB9127XOA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0516-9 269 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 02/01/2009 2 NDC:69968-0516-6 177 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 02/01/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 02/01/2009 Labeler - Johnson & Johnson Consumer Inc. (118772437)