VISICOL- sodium phosphate, monobasic, monohydrate and sodium phosphate, dibasic, anhydrous tablet
Salix Pharmaceuticals, Inc
----------
WARNINGS
There have been rare, but serious reports of acute phosphate nephropathy in patients who received oral sodium phosphate products for colon cleansing prior to colonoscopy. Some cases have resulted in permanent impairment of renal function and some patients required long-term dialysis. While some cases have occurred in patients without identifiable risk factors, patients at increased risk of acute phosphate nephropathy may include those with increased age, hypovolemia, increased bowel transit time (such as bowel obstruction), active colitis, or baseline kidney disease, and those using medicines that affect renal perfusion or function (such as diuretics, angiotensin converting enzyme [ACE] inhibitors, angiotensin receptor blockers [ARBs], and possibly nonsteriodal anti-inflammatory drugs [NSAIDs]).
See WARNINGS.
It is important to use the dose and dosing regimen as recommended (pm/am split dose).
See DOSAGE and ADMINISTRATION.
DESCRIPTION
Visicol® (sodium phosphate monobasic monohydrate, USP, and sodium phosphate dibasic anhydrous, USP) is a purgative used to clean the colon prior to colonoscopy. Visicol Tablets are white to off-white compressed tablets, with a monogram "I" on each side of the upper surface and a plain lower surface. Each tablet contains 1.102 grams of sodium phosphate monobasic monohydrate, USP and 0.398 grams of sodium phosphate dibasic anhydrous, USP for a total of 1.5 grams of sodium phosphate per tablet. Inert ingredients include microcrystalline cellulose (MCC), NF; magnesium stearate, NF; and colloidal silicon dioxide, NF. Visicol is gluten-free.
The structural and molecular formulae and molecular weights of the active ingredients are shown below:
- •
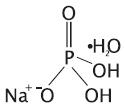
- Sodium phosphate monobasic monohydrate, USP
- •
-
Molecular Formula: NaH2PO4•H2O
Molecular Weight: 137.99 - •
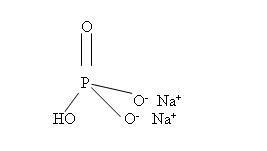
- Sodium phosphate dibasic anhydrous, USP
- •
-
Molecular Formula: Na2HPO4
Molecular Weight: 141.96
Visicol Tablets are for oral administration only.
CLINICAL PHARMACOLOGY
Visicol Tablets, taken in two doses of 30 grams (the complete regimen contains a total of 60 grams of sodium phosphate) approximately twelve hours apart, induces diarrhea, which effectively cleanses the entire colon. Each administration has a purgative effect for approximately 1 to 3 hours. The primary mode of action is thought to be through osmotic action of sodium, causing large amounts of water to be drawn into the colon, promoting colon evacuation.
Pharmacokinetics
An open-label pharmacokinetic study of Visicol in healthy volunteers was performed to determine the concentration-time profile of serum inorganic phosphorus levels after Visicol administration. All subjects received a total of 60 grams of sodium phosphate with a total liquid volume of 3.6 quarts. Subjects received a 30 gram dose (20 tablets given as 3 tablets every 15 minutes with 8 ounces of clear liquids) beginning at 6 PM and then received a second 30 gram dose (20 tablets given as 3 tablets every 15 minutes with 8 ounces of clear liquids) the following morning beginning at 6 AM.
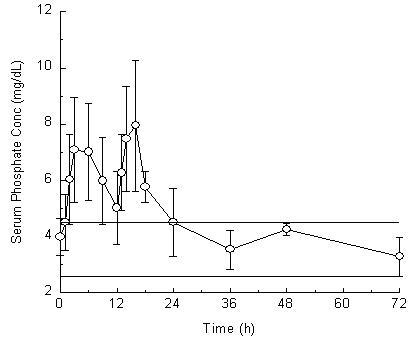
Twenty-three healthy subjects (mean age 57 years old; 57% male and 43% female; and 65% Hispanic, 30% Caucasian, and 4% African-American) participated in this pharmacokinetic study. The serum phosphorus level rose from a mean (± standard deviation) baseline of 4.0 (± 0.7) mg/dL to 7.7 (± 1.6 mg/dL), at a median of 3 hours after the administration of the first 30 gram dose of Visicol Tablets (see Figure 1).
Figure 1. Mean (± standard deviation) serum phosphorus concentrations
The serum phosphorus level rose to a mean of 8.4 (± 1.9) mg/dL, at a median of 4 hours after the administration of the second 30 gram dose of Visicol Tablets. The serum phosphorus level remained above baseline for a median of 24 hours after the administration of the initial dose of Visicol Tablets (range 16 to 48 hours).
The upper (4.5 mg/dL) and lower (2.6 mg/dL) reference limits for serum phosphate are represented by solid bars.
Special Populations
Renal insufficiency: The effect of renal dysfunction on Visicol Tablets pharmacokinetics has not been studied. Since the inorganic form of phosphate in the circulating plasma is excreted almost entirely by the kidneys, patients with renal disease may have difficulty excreting a large phosphate load. Thus, Visicol Tablets should be used with caution in patients with impaired renal function (see WARNINGS).
Hepatic insufficiency: Visicol Tablets have not been investigated in patients with hepatic failure. Visicol is not expected to be metabolized in the liver.
Geriatric: In a single pharmacokinetic study of sodium phosphate tablets, which included 6 elderly volunteers, plasma half-life increased two-fold in subjects > 70 years of age compared to subjects < 50 years of age (3 subjects and 5 subjects, respectively).
Gender: No difference in serum phosphate AUC values were observed in the single pharmacokinetic study conducted with Visicol in 13 male and 10 female healthy volunteers.
CLINICAL STUDIES
A total of 957 adult patients were enrolled and treated in the controlled clinical trials of Visicol Tablets. Males and females were about equally represented. Approximately 87% of the study population was Caucasian. Visicol Tablets were found to be comparable in cleansing efficacy to the comparison drug, a commercially available polyethylene glycol-salt (PEG-salt solution) solution (Cherry Flavor NuLYTELY®). Two identical, single (investigator) blind, randomized, multicenter trials were conducted comparing the efficacy and safety of Visicol Tablets and the PEG-salt solution comparator as a colon cleansing agent in patients undergoing routine diagnostic colonoscopy. In each study, over 200 patients were randomized to self-administer the Visicol Tablets and over 200 were randomized to self-administer the PEG-salt solution comparator. Colonoscopy was generally performed within 5 hours of the second dose. Physicians used a four-point, validated Physician Questionnaire to assess efficacy. The distribution of “excellent”, “good”, “fair” and “inadequate”, as evaluated by the physician performing the colonoscopy, was comparable in both groups. Cleansing efficacy observed in these studies is described in Table 1.
| Efficacy Rating | Study A | Study B | ||
|---|---|---|---|---|
| Viscol Tablets
n (%) | PEG-salt solution Comparator
n (%) | Viscol Tablets
n (%) | PEG-salt solution Comparator
n (%) |
|
|
† p values (Cochran-Mantel-Haenszel Test) were calculated for comparisons between Excellent and Good versus Fair versus Inadequate; Visicol Tablets and PEG-salt solution comparator. |
||||
|
Excellent or Good |
171 (82.2) |
156 (75.4) |
183 (86.3) |
170 (78.0) |
|
Fair |
34 (16.3) |
49 (23.7) |
26 (12.3) |
45 (20.6) |
|
Inadequate |
3 (1.4) |
2 (1.0) |
3 (1.4) |
3 (1.4) |
|
Total patients |
208 |
207 |
212 |
218 |
|
p value † |
n.s. |
n.s. |
||
The efficacy of overall colonic cleansing with the Visicol Tablets was comparable to the PEG-salt solution. In addition, the incidence of “Inadequate” colon cleansing ratings due to poor purgative preparation was similar between Visicol Tablets and the PEG-salt solution comparator. Also, cleansing efficacy in the ascending colon with Visicol Tablets was comparable to the PEG-salt solution.
INDICATIONS AND USAGE
Visicol Tablets are indicated for cleansing of the colon as a preparation for colonoscopy in adults 18 years of age or older.
CONTRAINDICATIONS
Visicol Tablets are contraindicated in patients with biopsy-proven acute phosphate nephropathy.
Visicol Tablets are contraindicated in patients with a known allergy or hypersensitivity to sodium phosphate salts or any of its ingredients.
WARNINGS
Administration of sodium phosphate products prior to colonoscopy has resulted in fatalities due to significant fluid shifts, severe electrolyte abnormalities, and cardiac arrhythmias. These fatalities have been observed in patients with renal insufficiency, in patients with bowel perforation, and in patients who misused or overdosed sodium phosphate products. It is recommended that patients receiving Visicol be advised to adequately hydrate before, during, and after the use of Visicol.
Considerable caution should be advised before Visicol Tablets are used in patients with the following illnesses: severe renal insufficiency (creatinine clearance less than 30 mL/minute), congestive heart failure, ascites, unstable angina, acute bowel obstruction, bowel perforation, toxic megacolon, gastric retention, ileus, pseudo-obstruction of the bowel, severe chronic constipation, acute colitis, gastric bypass or stapling surgery or hypomotility syndrome.
Consider performing baseline and post-colonoscopy labs (phosphate, calcium, potassium, sodium, creatinine, and BUN) in patients who may be at increased risk for serious adverse events, including those with history of renal insufficiency, history of ─ or at greater risk of ─ acute phosphate nephropathy, known or suspected electrolyte disorders (such as dehydration), seizures, arrhythmias, cardiomyopathy, prolonged QT, recent history of a MI and those with known or suspected hyperphosphatemia, hypocalcemia, hypokalemia, and hypernatremia. Also if patients develop vomiting and/or signs of dehydration then measure post-colonoscopy labs (phosphate, calcium, potassium, sodium, creatinine, and BUN).
Renal Disease, Acute Phosphate Nephropathy, and Electrolyte Disorders
There have been rare, but serious reports of renal failure, acute phosphate nephropathy, and nephrocalcinosis in patients who received oral sodium phosphate products (including oral sodium phosphate solutions and tablets) for colon cleansing prior to colonoscopy. These cases often resulted in permanent impairment of renal function and several patients required long-term dialysis. The time to onset is typically within days; however, in some cases, the diagnosis of these events has been delayed up to several months after the ingestion of these products. Patients at increased risk of acute phosphate nephropathy may include patients with the following: hypovolemia, baseline kidney disease, increased age, and patients using medicines that affect renal perfusion or function [such as diuretics, angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers, and possibly nonsteroidal anti-inflammatory drugs (NSAIDs).
Use Visicol with caution in patients with impaired renal function, known or suspected electrolyte disturbances (such as dehydration), or people taking concomitant medications that may affect electrolyte levels (such as diuretics). Patients with electrolyte abnormalities such as hypernatremia, hyperphosphatemia, hypokalemia, or hypocalcemia should have them corrected before treatment with Visicol Tablets.
Seizures
There have been rare reports of generalized tonic-clonic seizures and/or loss of consciousness associated with use of sodium phosphate products in patients with no prior history of seizures. The seizure cases were associated with electrolyte abnormalities (e.g., hyponatremia, hypokalemia, hypocalcemia, and hypomagnesemia) and low serum osmolality. The neurologic abnormalities resolved with correction of fluid and electrolyte abnormalities. Visicol should be used with caution in patients with a history of seizures and in patients at higher risk of seizure [patients using concomitant medications that lower the seizure threshold (such as tricyclic antidepressants), patients withdrawing from alcohol or benzodiazepines, or patients with known or suspected hyponatremia].
Cardiac Arrhythmias
There have been rare, but serious reports of arrhythmias associated with the use of sodium phosphate products. Visicol should be used with caution in patients with higher risk of arrhythmias (patients with a history of cardiomyopathy, patients with prolonged QT, patients with a history of uncontrolled arrhythmias, and patients with a recent history of a myocardial infarction). Pre-dose and post-colonoscopy ECGs should be considered in patients with high risk of serious, cardiac arrhythmias.
PRECAUTIONS
General
Patients should be instructed to drink 8 ounces of clear liquids with each 3-tablet (or each 2-tablet) dose of Visicol. Patients should take a total of 3.6 quarts of clear liquids with Visicol. Inadequate fluid intake, as with any effective purgative, may lead to excessive fluid loss and hypovolemia. Dehydration from purgation may be exacerbated by inadequate oral fluid intake, vomiting, and/or the use of diuretics. Patients should not take additional laxatives or purgatives, particularly additional sodium phosphate-based products.
Prolongation of the QT interval has been observed in some patients who were dosed with Visicol Tablets. QT prolongation with Visicol Tablets has been associated with electrolyte imbalances, such as hypokalemia and hypocalcemia. Visicol Tablets should be used with caution in patients who are taking medications known to prolong the QT interval, since serious complications may occur. Pre-dose and post-colonoscopy ECGs should be considered in patients with known prolonged QT. In these studies, prolongation of the QT interval was also observed in some patients treated with PEG-salt solution.
Patients with a history of swallowing difficulties or anatomic narrowing of the esophagus, such as a stricture, may have difficulty swallowing Visicol Tablets. Undigested or partially digested Visicol Tablets may be seen in the stool or during colonoscopy. In addition, undigested tablets from other medications may be seen in the stool or during colonoscopy.
Administration of Visicol Tablets may induce colonic mucosal aphthous ulcerations, since this endoscopic finding observed with other sodium phosphate cathartic preparations. This colonoscopic finding should be considered in patients with known or suspect inflammatory bowel disease (IBD).
Because published data suggest that sodium phosphate absorption may be enhanced in patients experiencing an acute exacerbation of IBD, Visicol Tablets should be used with caution in IBD patients.
Since Visicol Tablets were not studied in patients who recently had cardiac surgery (including coronary artery bypass graft surgery) Visicol should be used with caution in these patients.
Drug Interactions
Medications administered in close proximity to Visicol Tablets may not be absorbed from the gastrointestinal tract due to the rapid intestinal peristalsis and watery diarrhea induced by the purgative agent.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been performed to evaluate the carcinogenic potential of Visicol. Studies to evaluate the effect of Visicol on fertility or its mutagenic potential have not been performed.
Pregnancy
Category C. Reproduction studies have not been conducted with Visicol. It is also not known whether Visicol can cause fetal harm when administered to a pregnant woman, or can affect reproduction capacity. Visicol Tablets should be given to a pregnant woman only if clearly needed.
Pediatric Use
Safety and efficacy of Visicol Tablets have not been demonstrated in patients less than 18 years of age.
Geriatric Use
Of the 980 subjects/patients in the Visicol studies, 284 (29%) subjects/patients were 65 years of age or older. Of the 548 subjects/patients who received Visicol in these studies, 146 (27%) were 65 years of age or older and 42 (8%) subjects/patients were 75 years of age or older.
In two phase 3 Visicol trials (Study A and Study B), no overall differences in safety or effectiveness were observed between geriatric patients and younger patients. Greater sensitivity of some older individuals cannot be ruled out; therefore, Visicol Tablets should be used with caution in geriatric patients.
Sodium phosphate is known to be substantially excreted by the kidney, and the risk of adverse reactions with sodium phosphate may be greater in patients with impaired renal function. Since geriatric patients are more likely to have impaired renal function, consider performing baseline and post-colonoscopy labs (phosphate, calcium, potassium, sodium, creatinine, and BUN) in these patients (see WARNINGS).
Adverse Reactions
In the phase 3 Visicol trials, bloating, nausea, abdominal pain, and vomiting were the most common drug-related adverse events reported with the use of Visicol (see Table 2). Since diarrhea was considered as a part of the efficacy of Visicol diarrhea was not defined as an adverse event in the clinical trials. Small superficial mucosal ulcerations, typical of those previously reported from the use of liquid preparations of sodium phosphate, and instances of mucosal bleeding have been observed on colonoscopy.
No patient in the clinical studies developed predefined postural changes in vital signs with concomitant symptoms of lightheadedness or syncope.
| Visicol ®
%=n/N | NuLYTELY ®
%=n/N |
|
|---|---|---|
| * Drug-related were adverse events possibly or probably drug-related | ||
|
N=427 |
N=432 |
|
|
Bloating |
47% |
61% |
|
Nausea |
35% |
54% |
|
Abdominal Pain |
30% |
36% |
|
Vomiting |
7% |
18% |
Electrolyte Changes
In Visicol trials, changes in serum electrolytes (including phosphate, calcium, potassium, and sodium) have been observed in patients taking Visicol Tablets.
In the Visicol phase 3 trials, 96% and <1% of patients who took Visicol (60 grams) and NuLYTELY (up to 4 liters), respectively, developed hyperphosphatemia (defined as phosphate level > 4.7 mg/dL) on the day of the colonoscopy. In these trials, patients who took Visicol and NuLYTELY had baseline mean phosphate levels of 3.3 and 3.4 mg/dL and subsequently developed on the day of the colonoscopy mean phosphate levels of 7.1 and 3.3 mg/dL, respectively.
Two to three days after colonoscopy, 34%, 66%, and 0% of patients who received Visicol had (reactive) hypophosphatemia (defined as phosphate level < 2.4 mg/dL), normal phosphate levels, and hyperphosphatemia, respectively. Two to three days after colonoscopy, 3%, 96%, and 1% of patients who received NuLYTELY had (reactive) hypophosphatemia, normal phosphate levels, and hyperphosphatemia, respectively. Two to three days after colonoscopy, patients who took Visicol and NuLYTELY had mean phosphate levels of 2.6 and 3.3 mg/dL, respectively.
In the Visicol phase 3 trials, 47% and 9% of patients who took Visicol and NuLYTELY, respectively, developed hypocalcemia (defined as calcium level < 8.6 mg/dL) on the day of the colonoscopy. The mean changes in calcium levels (from baseline) for the Visicol and NuLYTELY patients were -0.6 and -0.1 mg/dL, respectively. Furthermore, in these trials, 28% and 3% of patients who took Visicol and NuLYTELY, respectively, developed hypokalemia (defined as potassium level < 3.5 mEq/L) on the day of the colonoscopy. The mean changes in potassium levels (from baseline) for the Visicol and NuLYTELY patients were -0.5 and -0.1 mEq/L, respectively. None of the patients who developed hypocalcemia or hypokalemia in the trials required treatment.
Postmarketing Experience
In addition to adverse events reported from clinical trials, the following adverse events have been identified during post-approval use of Visicol. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to either their seriousness, frequency of reporting or causal connection to Visicol, or a combination of these factors.
General: Hypersensitivity reactions including anaphylaxis, rash, pruritus, urticaria, throat tightness, bronchospasm, dyspnea, pharyngeal edema, dysphagia, paresthesia and swelling of the lips and tongue, and facial swelling.
Cardiovascular: Arrhythmias
Nervous system: Seizures
Renal: Renal impairment, increased blood urea nitrogen (BUN), increased creatinine, acute renal failure, acute phosphate nephropathy, nephrocalcinosis, and renal tubular necrosis
OVERDOSAGE
There have been no reported cases of overdosage with Visicol Tablets. Purposeful or accidental ingestion of more than the recommended dosage of Visicol Tablets might be expected to lead to severe electrolyte disturbances, including hyperphosphatemia, hypocalcemia, hypernatremia, or hypokalemia, as well as dehydration and hypovolemia, with attendant signs and symptoms of these disturbances. Certain severe electrolyte disturbances may lead to cardiac arrhythmias, seizure, renal failure, and death. The patient who has taken an overdosage should be monitored carefully, and treated symptomatically for complications until stable.
DOSAGE AND ADMINISTRATION
The recommended dose of Visicol Tablets for colon cleansing for adult patients is 40 tablets (60 grams of sodium phosphate) taken orally with a total of 3.6 quarts of clear liquids in the following manner:
The evening before the colonoscopy procedure: Take 3 Visicol Tablets (the last dose will be 2 Visicol Tablets) with 8 ounces of clear liquids every 15 minutes for a total of 20 tablets.
On the day of the colonoscopy procedure: Starting 3-5 hours before the procedure, take 3 Visicol Tablets (the last dose will be 2 Visicol Tablets) with 8 ounces of clear liquids every 15 minutes for a total of 20 tablets.
It is recommended that patients receiving Visicol be advised to adequately hydrate before, during, and after the use of Visicol.
Patients should not use Visicol within seven days of previous administration. No additional enema or laxative is required, and patients should be advised NOT to take additional agents, particularly those containing sodium phosphate.
HOW SUPPLIED
Visicol Tablets are supplied in child-resistant bottles containing 40 tablets and 100 tablets. Each tablet contains 1.102 g sodium phosphate monobasic monohydrate, USP and 0.398 g sodium phosphate dibasic anhydrous, USP for a total of 1.5 g of sodium phosphate per tablet. Each bottle contains two silica desiccant packets, which are not to be ingested.
NDC 65649-601-04 (40 tablets)
NDC 65649-601-41 (100 tablets)
Rx only
Storage
Store at 25°C (77°F); excursions permitted to 15-30°C (59-86°F) [See USP Controlled Room Temperature]. Discard any unused portion.
Manufactured by:
Pharmaceutical Manufacturing Research Services Inc.
Horsham, PA 19044
For:
Salix Pharmaceuticals, Inc.
Morrisville, NC 27560
© 2005 Salix Pharmaceuticals, Inc.
VENART-110-2/Mar 2009
Product protected by U.S. Patent No. 5,616,346 and other pending applications.
Medication Guide
VISICOL® (VĬ-zə-käl) (sodium phosphate monobasic monohydrate, USP and sodium phosphate dibasic anhydrous, USP) Tablets
Read the Medication Guide that comes with VISICOL before you start taking it and each time you take it. This Medication Guide does not take the place of talking with your doctor about your condition or your treatment. If you have any questions about VISICOL, ask your doctor or pharmacist.
What is the most important information I should know about VISICOL?
VISICOL can cause serious side effects, including:
Serious kidney problems. Rare, but serious kidney problems can happen in people who take medicines made with sodium phosphate, including VISICOL, to clean your colon before a colonoscopy. These kidney problems can sometimes lead to kidney failure or the need for dialysis for a long time. These problems often happen within a few days, but sometimes may happen several months after taking VISICOL.
Conditions that can make you more at risk for having serious kidney problems with VISICOL include if you:
- •
- lose too much body fluid (dehydration)
- •
- have slow moving bowels
- •
- have bowels blocked with stool (constipation)
- •
- have severe stomach pain or bloating
- •
- have any disease that causes bowel irritation (colitis)
- •
- have kidney disease
- •
- have heart failure
- •
- take water pills or non-steroidal anti-inflammatory drugs (NSAIDS).
Your age may also affect your risk for having kidney problems with VISICOL.
Before you start taking VISICOL, tell your doctor if you:
- •
- have any kidney problems.
- •
- take any medicines for blood pressure, heart disease or kidney disease.
Severe fluid loss (dehydration). People who take medicines that contain sodium phosphate can have severe loss of body fluid, with severe changes in body salts in the blood, and abnormal heart rhythms. These problems can lead to death.
Tell your doctor if you have any of these symptoms of loss of too much body fluid (dehydration) while taking VISICOL:
- •
- vomiting
- •
- dizziness
- •
- urinating less often than normal
- •
- headache
See “What are the possible side effects of VISICOL?” for more information about side effects.
What is VISICOL?
VISICOL is a prescription medicine used in adults 18 years and older, to clean your colon before a colonoscopy. VISICOL cleans your colon by causing you to have diarrhea. Cleaning your colon helps your doctor see the inside of your colon more clearly during the colonoscopy.
It is not known if VISICOL is safe and works in children under age 18.
Who should not take VISICOL?
Do not take VISICOL if:
- •
- you have had a kidney biopsy that shows you have kidney problems because of too much phosphate
- •
- you are allergic to sodium phosphate salts or any of the ingredients in VISICOL. See the end of this Medication Guide for a list of ingredients in Visicol.
What should I tell my doctor before taking VISICOL?
Before taking VISICOL, tell your doctor about all of your medical conditions, including if you have:
- •
- any of the medical conditions listed in the section “What is the most important information I should know about VISICOL?”
- •
- irritation of the bowel (colitis). VISICOL can cause symptoms of irritable bowel disease to flare-up.
- •
- damage to your bowels
- •
- had stomach surgery
- •
- trouble swallowing pills. You may have trouble swallowing VISICOL.
- •
- problems with an abnormal heart beat
- •
- had a recent heart attack or have other heart problems
- •
- symptoms of too much body fluid loss (dehydration) including vomiting, dizziness, urinating less often than normal, or headache
- •
- a history of seizures
- •
- if you drink alcohol
- •
- recently had coronary artery bypass graft surgery (CABG)
- •
- you are on a low salt diet
- •
- are pregnant. It is not known if VISICOL will harm your unborn baby.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. Any medicine that you take close to the time that you take VISICOL may not work as well. Especially tell your doctor if you take:
- •
- water pills (diuretics)
- •
- medicines for blood pressure or heart problems
- •
- medicines for kidney damage
- •
- medicines for pain, such as aspirin or a non-steroidal anti-inflammatory drug (NSAID)
- •
- a medicine for seizures
- •
- antidepressant medicines or a medicine for anxiety
- •
- a laxative for constipation in the last 7 days. You should not take another medicine that contains sodium phosphate while you take VISICOL.
Ask your doctor if you are not sure if your medicine is one that is listed above.
Know the medicines you take. Keep a list of your medicines to show your doctor or pharmacist when you get a new prescription.
How should I take VISICOL?
- •
- Take VISICOL exactly as prescribed by your doctor.
- •
- You will take VISICOL on the evening before your colonoscopy and about 12 hours later, on the day of your colonoscopy, as described below.
- •
- It is important for you to drink clear liquids before, during, and after taking VISICOL. This may help prevent kidney damage. Examples of clear liquids are water, flavored water, lemonade (no pulp), ginger ale, or apple juice. Do not drink any liquids colored purple or red.
- •
- You must read, understand, and follow these instructions to take VISICOL the right way:
On the evening before your colonoscopy, you will take a total of 20 VISICOL tablets, as follows:
- 1.
- Take 3 VISICOL tablets with 8 ounces of clear liquids.
- 2.
- Wait 15 minutes.
- 3.
- Take 3 more VISICOL tablets with 8 ounces of clear liquids.
- 4.
- Repeat steps 2 and 3 above four more times. Make sure you wait 15 minutes after each time.
- 5.
- When you take your last dose of VISICOL, the dose will be 2 tablets with 8 ounces of clear liquids.
On the day of your colonoscopy, you will take another 20 VISICOL Tablets
about, 3-5 hours before your colonoscopy, as follows:
- 1.
- Take 3 VISICOL tablets with 8 ounces of clear liquids.
- 2.
- Wait 15 minutes.
- 3.
- Take 3 more VISICOL tablets with 8 ounces of clear liquids.
- 4.
- Repeat steps 2 and 3 above four more times. Make sure you wait 15 minutes after each time.
- 5.
- When you take your last dose of VISICOL, the dose will be 2 tablets with 8 ounces of clear liquids.
Tell your doctor if you have any of these symptoms while taking VISICOL:
- •
- vomiting, dizziness, or if you urinate less often than normal. These may be signs that you have lost too much fluid while taking VISICOL.
- •
- trouble drinking clear fluids
- •
- severe stomach cramping, bloating, nausea, or headache.
If you take too much VISICOL, call your doctor or get medical help right away.
What should I avoid while taking VISICOL?
- •
- You should not take other laxatives or enemas, especially those made with sodium phosphate, while taking VISICOL.
- •
- You should not use VISICOL if you have already used it in the last 7 days.
What are the possible side effects of VISICOL?
Visicol can cause serious side effects, including:
- •
- See “What is the most important information I should know about VISICOL?”
- •
- seizures or fainting (black-outs). People who take a medicine that contains sodium phosphate, such as VISICOL, can have seizures or faint (become unconscious) even if they have not had seizures before. Tell your doctor right away if you have a seizure or faint while taking VISICOL. See “What should I tell my doctor before taking VISICOL?”
- •
- abnormal heart beat (arrhythmia)
- •
- changes in your blood levels of calcium, phosphate, potassium, sodium
The most common side effects of VISICOL are:
- •
- bloating
- •
- stomach area (abdominal) pain
- •
- nausea
- •
- vomiting
These are not all the possible side effects of VISICOL. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How do I store VISICOL?
- •
- Store VISICOL at room temperature, between 59° F to 86° F(15° C to 30° C).
- •
- Throw away any VISICOL that is not needed.
- •
- Keep VISICOL and all medicines out of the reach of children.
General information about VISICOL
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use VISICOL for a condition for which it was not prescribed. Do not give VISICOL to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about VISICOL. If you would like more information about VISICOL, talk with your doctor or pharmacist. You can ask your doctor or pharmacist for information that is written for healthcare professionals. For more information, call 1-866-669-7597 (toll-free) or go to: www.Salix.com.
What are the ingredients in VISICOL?
Active ingredients: sodium phosphate monobasic monohydrate and sodium phosphate dibasic anhydrous
Inactive ingredients: microcrystalline cellulose, magnesium stearate, and colloidal silicon dioxide
Salix Pharmaceuticals, Inc.
Morrisville, NC 27560, USA
Revised March 2009
This Medication Guide has been approved by the U.S. Food and Drug Administration.
VENART-110-2/Mar 2009
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - Visicol 40 Tablet, Bottle Label
NDC 65649-601-04
Rx only
40 Tablets
Visicol®
(sodium phosphate monobasic monohydrate, USP
and sodium phosphate dibasic anhydrous, USP)
TABLETS
Dispense the accompanying Medication Guide to each patient
Salix Pharmaceuticals, Inc.
| VISICOL
sodium phosphate, monobasic, monohydrate, and sodium phosphate, dibasic anhydrous tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Salix Pharmaceuticals, Inc (793108036) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmaceutical Manufacturing Research Services Inc. | 836225649 | MANUFACTURE(65649-601) | |