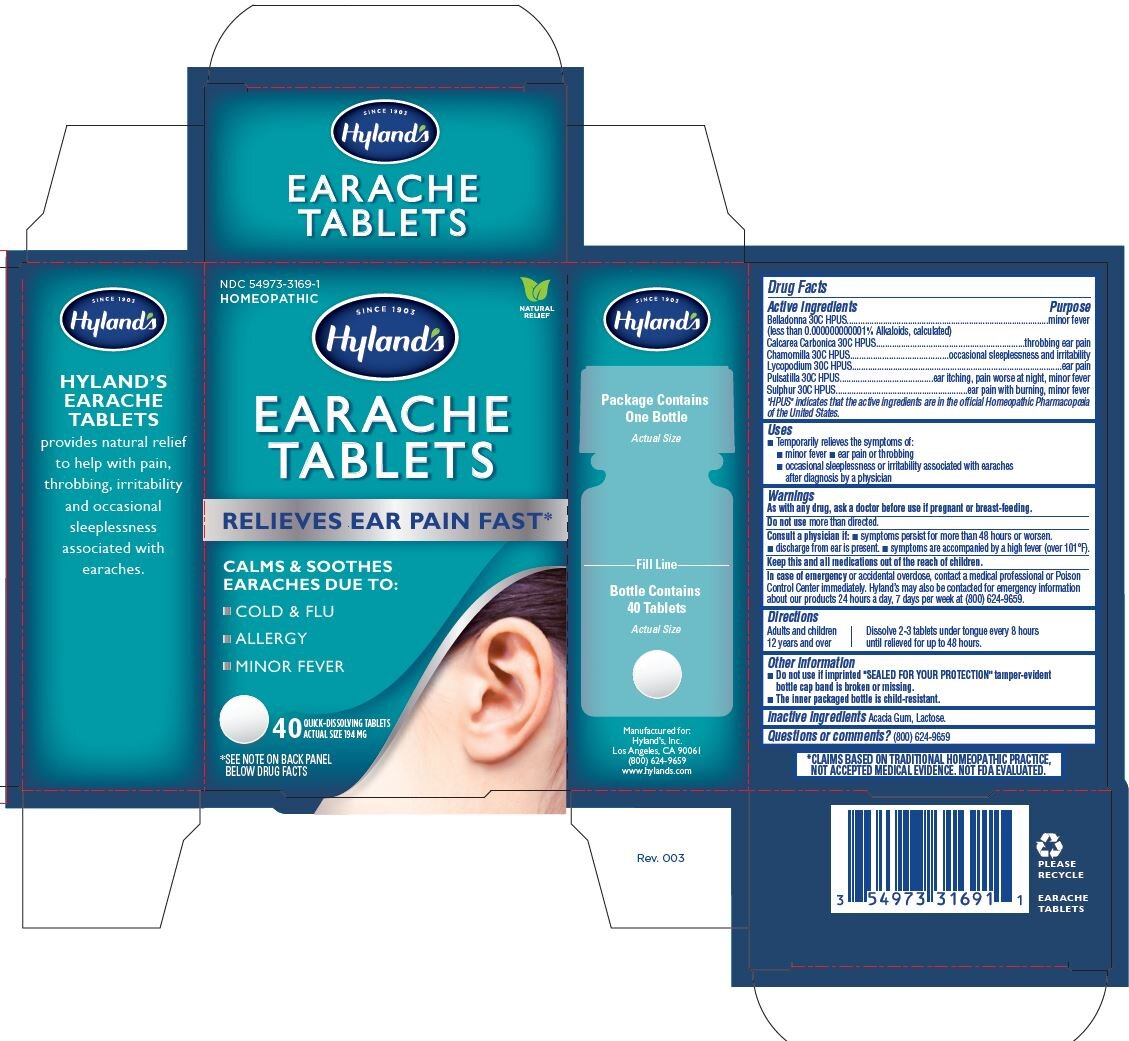
EARACHE- matricaria chamomilla, atropa belladonna, oyster shell calcium carbonate, crude, sulfur, pulsatilla vulgaris and lycopodium clavatum spore tablet
Hyland's
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Hyland's Earache Tablets
Drug Facts
|
Active ingredients |
Purpose |
|
Belladonna 30C HPUS |
minor fever |
|
(less than 0.000000000001% Alkaloids, calculated) | |
|
Calcarea Carbonica 30C HPUS |
throbbing ear pain |
|
Chamomilla 30C HPUS |
occasional sleeplessness and irritability |
|
Lycopodium 30C HPUS |
ear pain |
|
Pulsatilla 30C HPUS |
ear itching, pain worse at night,minor fever |
|
Sulphur 30C HPUS |
ear pain with burning,minor fever |
“HPUS” indicates that the active ingredients are in the official
Homeopathic Pharmacopoeia of the United States.
Uses
- Temporarily relieves the symptoms of:
- minor fever
- ear pain or throbbing
- occasional sleeplessness or irritability associated with earaches after diagnosis by a physician
Warnings
As with any drug, ask a doctor before use if pregnant or breast-feeding.
Directions
|
Adults and children 12 years and over |
Dissolve 2-3 tablets under tongue every 8 hours until relieved for up to 48 hours. |
Other information
- Do not use if imprinted "SEALED FOR YOUR PROTECTION" tamper-evident
bottle cap band is broken or missing.
- The inner packaged bottle is child-resistant.
| EARACHE
matricaria chamomilla, atropa belladonna, oyster shell calcium carbonate, crude, sulfur, pulsatilla vulgaris and lycopodium clavatum spore tablet |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Hyland's (028570695) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Standard Homeopathic Company | 008316655 | manufacture(54973-3169) , pack(54973-3169) , label(54973-3169) | |