Label: HYDROCODONE POLISTIREX AND CHLORPHENIRAMINE POLISITREX suspension, extended release
-
Contains inactivated NDC Code(s)
NDC Code(s): 17856-0235-1 - Packager: Atlantic Biologicals Corps
- This is a repackaged label.
- Source NDC Code(s): 49884-235
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CII
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 17, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Each 5 mL of hydrocodone polistirex and chlorpheniramine polistirex extended release (ER) oral suspension contains hydrocodone polistirex equivalent to 10 mg of hydrocodone bitartrate and chlorpheniramine polistirex equivalent to 8 mg of chlorpheniramine maleate. Hydrocodone is a centrally-acting narcotic antitussive. Chlorpheniramine is an antihistamine. Hydrocodone polistirex and chlorpheniramine polistirex ER oral suspension is for oral use only.
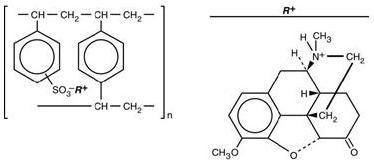
Hydrocodone Polistirex
Sulfonated styrene-divinylbenzene copolymer complex with 4,5α-epoxy-3-methoxy-17-methylmorphinan-6-one.
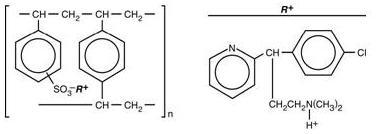
Chlorpheniramine Polistirex
Sulfonated styrene-divinylbenzene copolymer complex with 2-[p-chloro-α-[2-(dimethylamino)ethyl]-benzyl]pyridine.
Inactive Ingredients
Ascorbic acid, D&C Yellow No. 10, flavors, high fructose corn syrup, modified food starch, methylparaben, polysorbate 80, polyvinyl acetate, propylene glycol, propylparaben, purified water, sodium ascorbate, sodium metabisulfite, sodium polystyrene sulfonate, sucrose, triacetin, xanthan gum.
-
CLINICAL PHARMACOLOGY
Hydrocodone is a semisynthetic narcotic antitussive and analgesic with multiple actions qualitatively similar to those of codeine. The precise mechanism of action of hydrocodone and other opiates is not known; however, hydrocodone is believed to act directly on the cough center. In excessive doses, hydrocodone, like other opium derivatives, will depress respiration. The effects of hydrocodone in therapeutic doses on the cardiovascular system are insignificant. Hydrocodone can produce miosis, euphoria, and physical and psychological dependence.
Chlorpheniramine is an antihistamine drug (H receptor antagonist) that also possesses anticholinergic and sedative activity. It prevents released histamine from dilating capillaries and causing edema of the respiratory mucosa. 1
Hydrocodone release from hydrocodone polistirex and chlorpheniramine polistirex ER oral suspension is controlled by an extended-release drug delivery system, which combines an ion-exchange polymer matrix with a diffusion rate-limiting permeable coating. Chlorpheniramine release is prolonged by use of an ion-exchange polymer system.
Following multiple dosing with hydrocodone polistirex and chlorpheniramine polistirex ER oral suspension, hydrocodone mean (S.D.) peak plasma concentrations of 22.8 (5.9) ng/mL occurred at 3.4 hours. Chlorpheniramine mean (S.D.) peak plasma concentrations of 58.4 (14.7) ng/mL occurred at 6.3 hours following multiple dosing. Peak plasma levels obtained with an immediate-release syrup occurred at approximately 1.5 hours for hydrocodone and 2.8 hours for chlorpheniramine. The plasma half-lives of hydrocodone and chlorpheniramine have been reported to be approximately 4 and 16 hours, respectively.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Hydrocodone polistirex and chlorpheniramine polistirex extended-release oral suspension is contraindicated in patients with a known allergy or sensitivity to hydrocodone or chlorpheniramine.
The use of hydrocodone polistirex and chlorpheniramine polistirex ER oral suspension is contraindicated in children less than 6 years of age due to the risk of fatal respiratory depression.
-
WARNINGS
Contains sodium metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
Respiratory Depression
As with all narcotics, hydrocodone polistirex and chlorpheniramine polistirex ER oral suspension produces dose-related respiratory depression by directly acting on brain stem respiratory centers. Hydrocodone affects the center that controls respiratory rhythm and may produce irregular and periodic breathing. Caution should be exercised when hydrocodone polistirex and chlorpheniramine polistirex ER oral suspension is used postoperatively and in patients with pulmonary disease, or whenever ventilatory function is depressed. If respiratory depression occurs, it may be antagonized by the use of naloxone hydrochloride and other supportive measures when indicated (see ). OVERDOSAGE
Head Injury and Increased Intracranial Pressure
The respiratory depressant effects of narcotics and their capacity to elevate cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury, other intracranial lesions, or a pre-existing increase in intracranial pressure. Furthermore, narcotics produce adverse reactions, which may obscure the clinical course of patients with head injuries.
Acute Abdominal Conditions
The administration of narcotics may obscure the diagnosis or clinical course of patients with acute abdominal conditions.
Obstructive Bowel Disease
Chronic use of narcotics may result in obstructive bowel disease especially in patients with underlying intestinal motility disorder.
Pediatric Use
The use of hydrocodone polistirex and chlorpheniramine polistirex ER oral suspension is contraindicated in children less than 6 years of age (see ). CONTRAINDICATIONS
In pediatric patients, as well as adults, the respiratory center is sensitive to the depressant action of narcotic cough suppressants in a dose-dependent manner. Caution should be exercised when administering hydrocodone polistirex and chlorpheniramine polistirex ER oral suspension to pediatric patients 6 years of age and older. Overdose or concomitant administration of hydrocodone polistirex and chlorpheniramine polistirex ER oral suspension with other respiratory depressants may increase the risk of respiratory depression in pediatric patients. Benefit to risk ratio should be carefully considered, especially in pediatric patients with respiratory embarrassment (e.g., croup) (see ). PRECAUTIONS
-
PRECAUTIONS
General
Caution is advised when prescribing this drug to patients with narrow-angle glaucoma, asthma, or prostatic hypertrophy.
Special Risk Patients
As with any narcotic agent, hydrocodone polistirex and chlorpheniramine polistirex ER oral suspension should be used with caution in elderly or debilitated patients and those with severe impairment of hepatic or renal function, hypothyroidism, Addison's disease, prostatic hypertrophy, or urethral stricture. The usual precautions should be observed and the possibility of respiratory depression should be kept in mind.
Information for Patients
Patients should be advised that hydrocodone polistirex and chlorpheniramine polistirex ER oral suspension may produce marked drowsiness and impair the mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery.
Hydrocodone polistirex and chlorpheniramine polistirex ER oral suspension must not be diluted with fluids or mixed with other drugs as this may alter the resin-binding and change the absorption rate, possibly increasing the toxicity.
Patients should be advised to measure hydrocodone polistirex and chlorpheniramine polistirex ER oral suspension with an accurate measuring device. A household teaspoon is not an accurate measuring device and could lead to overdosage. A pharmacist can recommend an appropriate measuring device and can provide instructions for measuring the correct dose.
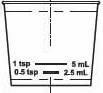
The dosing cup is provided with the 4 fl. oz. (115 mL) packaged product. The dosing cup fills for a 2.5 mL and for a 5 mL dose. Instruct the patient to fill to the line for the dose that has been prescribed. Rinse the measuring device after each use.
Shake well before using.
Keep out of the reach of children.
Cough Reflex
Hydrocodone suppresses the cough reflex; as with all narcotics, caution should be exercised when hydrocodone polistirex and chlorpheniramine polistirex ER oral suspension is used postoperatively, and in patients with pulmonary disease.
Drug Interactions
Patients receiving narcotics, antihistaminics, antipsychotics, antianxiety agents, or other CNS depressants (including alcohol) concomitantly with hydrocodone polistirex and chlorpheniramine polistirex ER oral suspension may exhibit an additive CNS depression. When combined therapy is contemplated, the dose of one or both agents should be reduced.
The use of MAO inhibitors or tricyclic antidepressants with hydrocodone preparations may increase the effect of either the antidepressant or hydrocodone.
The concurrent use of other anticholinergics with hydrocodone may produce paralytic ileus.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity, mutagenicity, and reproductive studies have not been conducted with hydrocodone polistirex and chlorpheniramine polistirex ER oral suspension.
PREGNANCY
Teratogenic Effects – Pregnancy Category C
Hydrocodone has been shown to be teratogenic in hamsters when given in doses 700 times the human dose. There are no adequate and well-controlled studies in pregnant women. Hydrocodone polistirex and chlorpheniramine polistirex ER oral suspension should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects
Babies born to mothers who have been taking opioids regularly prior to delivery will be physically dependent. The withdrawal signs include irritability and excessive crying, tremors, hyperactive reflexes, increased respiratory rate, increased stools, sneezing, yawning, vomiting, and fever. The intensity of the syndrome does not always correlate with the duration of maternal opioid use or dose.
Labor and Delivery
As with all narcotics, administration of hydrocodone polistirex and chlorpheniramine polistirex ER oral suspension to the mother shortly before delivery may result in some degree of respiratory depression in the newborn, especially if higher doses are used.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from hydrocodone polistirex and chlorpheniramine polistirex ER oral suspension, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
The use of hydrocodone polistirex and chlorpheniramine polistirex ER oral suspension is contraindicated in children less than 6 years of age (see and ). CONTRAINDICATIONSADVERSE REACTIONS, Respiratory, Thoracic and Mediastinal Disorders
Hydrocodone polistirex and chlorpheniramine polistirex ER oral suspension should be used with caution in pediatric patients 6 years of age and older (see ). WARNINGS, Pediatric Use
Geriatric Use
Clinical studies of hydrocodone polistirex and chlorpheniramine polistirex ER oral suspension did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
-
ADVERSE REACTIONS
Gastrointestinal Disorders
Nausea and vomiting may occur; they are more frequent in ambulatory than in recumbent patients. Prolonged administration of hydrocodone polistirex and chlorpheniramine polistirex ER oral suspension may produce constipation.
Nervous System Disorders
Sedation, drowsiness, mental clouding, lethargy, impairment of mental and physical performance, anxiety, fear, dysphoria, euphoria, dizziness, psychic dependence, mood changes.
Renal and Urinary Disorders
Ureteral spasm, spasm of vesical sphincters, and urinary retention have been reported with opiates.
Respiratory, Thoracic and Mediastinal Disorders
Dryness of the pharynx, occasional tightness of the chest, and respiratory depression (see ). CONTRAINDICATIONS
Hydrocodone polistirex and chlorpheniramine polistirex ER oral suspension may produce dose-related respiratory depression by acting directly on brain stem respiratory centers (see ). Use of hydrocodone polistirex and chlorpheniramine polistirex ER oral suspension in children less than 6 years of age has been associated with fatal respiratory depression. Overdose with hydrocodone polistirex and chlorpheniramine polistirex ER oral suspension in children 6 years of age and older, in adolescents, and in adults has been associated with fatal respiratory depression. OVERDOSAGE
DRUG ABUSE AND DEPENDENCE
Hydrocodone polistirex and chlorpheniramine polistirex extended-release oral suspension is a Schedule II narcotic. Psychic dependence, physical dependence and tolerance may develop upon repeated administration of narcotics; therefore, hydrocodone polistirex and chlorpheniramine polistirex ER oral suspension should be prescribed and administered with caution. However, psychic dependence is unlikely to develop when hydrocodone polistirex and chlorpheniramine polistirex ER oral suspension is used for a short time for the treatment of cough. Physical dependence, the condition in which continued administration of the drug is required to prevent the appearance of a withdrawal syndrome, assumes clinically significant proportions only after several weeks of continued oral narcotic use, although some mild degree of physical dependence may develop after a few days of narcotic therapy.
-
10 OVERDOSAGE
Signs and Symptoms
Serious overdosage with hydrocodone is characterized by respiratory depression (a decrease in respiratory rate and/or tidal volume, Cheyne-Stokes respiration, cyanosis), extreme somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, and sometimes bradycardia and hypotension. Although miosis is characteristic of narcotic overdose, mydriasis may occur in terminal narcosis or severe hypoxia. In severe overdosage apnea, circulatory collapse, cardiac arrest and death may occur. The manifestations of chlorpheniramine overdosage may vary from central nervous system depression to stimulation.
Treatment
Primary attention should be given to the reestablishment of adequate respiratory exchange through provision of a patent airway and the institution of assisted or controlled ventilation. The narcotic antagonist naloxone hydrochloride is a specific antidote for respiratory depression which may result from overdosage or unusual sensitivity to narcotics including hydrocodone. Therefore, an appropriate dose of naloxone hydrochloride should be administered, preferably by the intravenous route, simultaneously with efforts at respiratory resuscitation. Since the duration of action of hydrocodone in this formulation may exceed that of the antagonist, the patient should be kept under continued surveillance and repeated doses of the antagonist should be administered as needed to maintain adequate respiration. For further information, see full prescribing information for naloxone hydrochloride. An antagonist should not be administered in the absence of clinically significant respiratory depression. Oxygen, intravenous fluids, vasopressors and other supportive measures should be employed as indicated. Gastric emptying may be useful in removing unabsorbed drug.
-
DOSAGE AND ADMINISTRATION
(see ). It is important that hydrocodone polistirex and chlorpheniramine polistirex extended-release oral suspension is measured with an accurate measuring devicePRECAUTIONS, Information for Patients
The dosing cup is provided with the 4 fl. oz. (115 mL) packaged product. The dosing cup fills for a 2.5 mL and for a 5 mL dose. Instruct the patient to fill to the line for the dose that has been prescribed. Do not fill over the dose prescribed. Rinse with water after each use.
For prescriptions where a dosing device is not provided, a pharmacist can provide an appropriate measuring device and can provide instructions for measuring the correct dose. A household teaspoon is not an accurate measuring device and could lead to overdosage.
Each 5 mL of hydrocodone polistirex and chlorpheniramine polistirex ER oral suspension contains hydrocodone polistirex equivalent to 10 mg hydrocodone bitartrate, and chlorpheniramine polistirex equivalent to 8 mg chlorpheniramine maleate. Shake well before using. Rinse the measuring device with water after each use.
Shake well before using.
Children 6 to 11 Years of Age
2.5 mL every 12 hours; do not exceed 5 mL in 24 hours.
This medicine is contraindicated in children under 6 years of age (see ). CONTRAINDICATIONS
- HOW SUPPLIED
-
Patient Information
Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension (CII)
(10 mg hydrocodone bitartrate and 8 mg chlorpheniramine maleate per 5 mL)
Read this Patient Information before you start taking Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or your treatment.
What is Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension?
Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension is a prescription medicine used to treat cough and upper respiratory symptoms you can have with allergies or a cold. Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension is for adults and children age 6 years and older.
Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension contains two medicines, hydrocodone and chlorpheniramine. Hydrocodone is a narcotic cough suppressant. Chlorpheniramine is an antihistamine.
Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension is a controlled substance (CII) because it contains hydrocodone that can be a target for people who abuse prescription medicines or street drugs. Keep your Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension in a safe place, to protect it from theft. Never give your Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension to anyone else, because it may cause death or harm them. Selling or giving away this medicine is against the law.
Who should not take Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension?
Do not take Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension if you:
- •
- are allergic to any of the ingredients in Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension. See the end of this leaflet for a complete list of ingredients in Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension.
- •
- are a child under 6 years old. Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension can cause a decreased rate of breathing (respiratory depression) which can lead to death.
What should I tell my doctor before taking Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension?
Before you take Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension, tell your doctor if you:
- •
- have lung or breathing problems
- •
- have had a head injury
- •
- have pain in your belly (abdomen)
- •
- have eye problems, such as glaucoma
- •
- have prostate problems
- •
- have problems with your urinary tract (urethral stricture)
- •
- plan to have surgery
- •
- abuse alcohol
- •
- have kidney or liver problems
- •
- have thyroid problems, such as hypothyroidism
- •
- are pregnant or plan to become pregnant. If you take Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension regularly before your baby is born, your newborn baby may have withdrawal symptoms because their body has become use to the medicine.
Symptoms of withdrawal in a newborn baby may include:
- •
- irritability
- •
- crying more than usual
- •
- shaking (tremors)
- •
- jitteriness
- •
- breathing faster than normal
- •
- diarrhea or more stools than normal
- •
- sneezing
- •
- yawning
- •
- vomiting
- •
- fever
If you take Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension regularly before your baby is born, your baby could have breathing problems.
- •
- are breastfeeding or plan to breastfeed. You and your doctor should decide if you will take Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension or breastfeed. You should not do both.
- Tell your doctor about all of the medicines you take, including prescription and nonprescription medicines, vitamins, and herbal supplements.
- Using Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension with certain other medicines may affect each other. Using Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension with other medicines can cause serious side effects.
- Especially tell your doctor if you:
- •
- take pain medicines such as narcotics
- •
- take cold or allergy medicines that contain antihistamines or cough suppressants
- •
- take medicines for mental illness (anti-psychotics, anti-anxiety)
- •
- drink alcohol
- •
- take medicines for depression including monoamine oxidase inhibitors (MAOIs)
- •
- take medicines for stomach or intestine problems
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I take Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension?
- •
- Take Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension exactly as your doctor tells you.
- •
- Shake the Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension bottle well before you use it.
- •
- mix Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension with other fluids or medicines. Mixing may change how Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension works. Do not
- •
- Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension can be taken with or without food.
- •
- Only measure Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension with the dosing cup that comes with your prescription. See Figure A.
If you do not have a dosing cup for your medicine, ask your pharmacist to give you a measuring device to help you measure the correct amount of Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension. Do not use a household teaspoon to measure your medicine. You may accidently take too much.
- •
- The dosing cup is calibrated for measuring 2.5 mL and 5 mL dosages. Find the marked line for the dose you are taking.
- •
- Fill the cup so the medicine is leveled to the dose that has been prescribed. over the dosage line. Do not fill
- •
- Rinse the measuring cup with water after each use.
- Figure A
If you take too much Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension, call your doctor or go to the nearest hospital emergency room right away.
What should I avoid while taking Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension?
- •
- Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension can cause you to be drowsy. Do not drive a car or use machinery while you take Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension until you know how it affects you.
- •
- Do not drink alcohol while taking Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension. Drinking alcohol can increase your chances of having serious side effects.
What are the possible side effects of Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension?
Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension may cause serious side effects, including:
- •
- Decreased breathing (respiratory depression) which can lead to death. Call your doctor or get emergency treatment right away if you have:
- •
- shallow or slow breathing
- •
- confusion
- •
- excessive sleepiness
- •
- drowsiness leading to inability to think clearly or do normal physical activities
- •
- bowel problems, including constipation and bowel obstruction
The most common side effects of Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension are:
- •
- nausea and vomiting
- •
- constipation
- •
- nervous system problems (anxiety, fear, dizziness, mood changes)
- •
- urinary tract problems
- •
- dry throat
- •
- chest tightness
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
How should I store Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension?
- •
- Store Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension in a safe place at 68°F to 77°F (20°C to 25°C).
- •
- Safely throw away medicine that is out of date or no longer needed.
- •
- Keep Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension and all medicines out of the reach of children.
General information about Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information Leaflet. Do not use Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension for a condition for which it was not prescribed. Do not give Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension to other people, even if they have the same condition. It may harm them and it is against the law.
This Patient Information Leaflet summarizes the most important information about Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension that is written for healthcare professionals.
For more information about Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension, contact Par Pharmaceutical Inc. at 1-800-828-9393 or www.parpharm.com.
What are the ingredients in Hydrocodone Polistirex and Chlorpheniramine Polistirex Extended-Release Oral Suspension?
hydrocodone polistirex and chlorpheniramine polistirex Active Ingredient:
Ascorbic acid, D&C Yellow No. 10, flavors, high fructose corn syrup, modified food starch, methylparaben, polysorbate 80, polyvinyl acetate, propylene glycol, propylparaben, purified water, sodium ascorbate, sodium metabisulfite, sodium polystyrene sulfonate, sucrose, triacetin, xanthan gum. Inactive Ingredients:
This patient information has been approved by the U.S. Food and Drug Administration.
Distributed by:
Par Pharmaceutical Companies, Inc.
Spring Valley, NY 10977
I 09/14
- HYDROCODONE POLISTIREX AND CHLORPHENIRAMINE POLISITREX SUSPENSION, EXTENDED RELEASE
-
INGREDIENTS AND APPEARANCE
HYDROCODONE POLISTIREX AND CHLORPHENIRAMINE POLISITREX
hydrocodone polistirex and chlorpheniramine polisitrex suspension, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:17856-0235(NDC:49884-235) Route of Administration ORAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCODONE BITARTRATE (UNII: NO70W886KK) (HYDROCODONE - UNII:6YKS4Y3WQ7) HYDROCODONE BITARTRATE 10 mg in 5 mL CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 8 mg in 5 mL Inactive Ingredients Ingredient Name Strength SODIUM POLYSTYRENE SULFONATE (UNII: 1699G8679Z) WATER (UNII: 059QF0KO0R) VINYL ACETATE (UNII: L9MK238N77) TRIACETIN (UNII: XHX3C3X673) SODIUM METABISULFITE (UNII: 4VON5FNS3C) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) XANTHAN GUM (UNII: TTV12P4NEE) ASCORBIC ACID (UNII: PQ6CK8PD0R) SODIUM ASCORBATE (UNII: S033EH8359) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) SUCROSE (UNII: C151H8M554) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) MODIFIED CORN STARCH (1-OCTENYL SUCCINIC ANHYDRIDE) (UNII: 461P5CJN6T) Product Characteristics Color YELLOW Score Shape Size Flavor PINEAPPLE (PEACH) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17856-0235-1 5 mL in 1 CUP, UNIT-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091632 08/16/2012 Labeler - Atlantic Biologicals Corps (047437707) Registrant - Atlantic Biologicals Corps (047437707) Establishment Name Address ID/FEI Business Operations Atlantic Biologicals Corps 047437707 RELABEL(17856-0235) , REPACK(17856-0235)