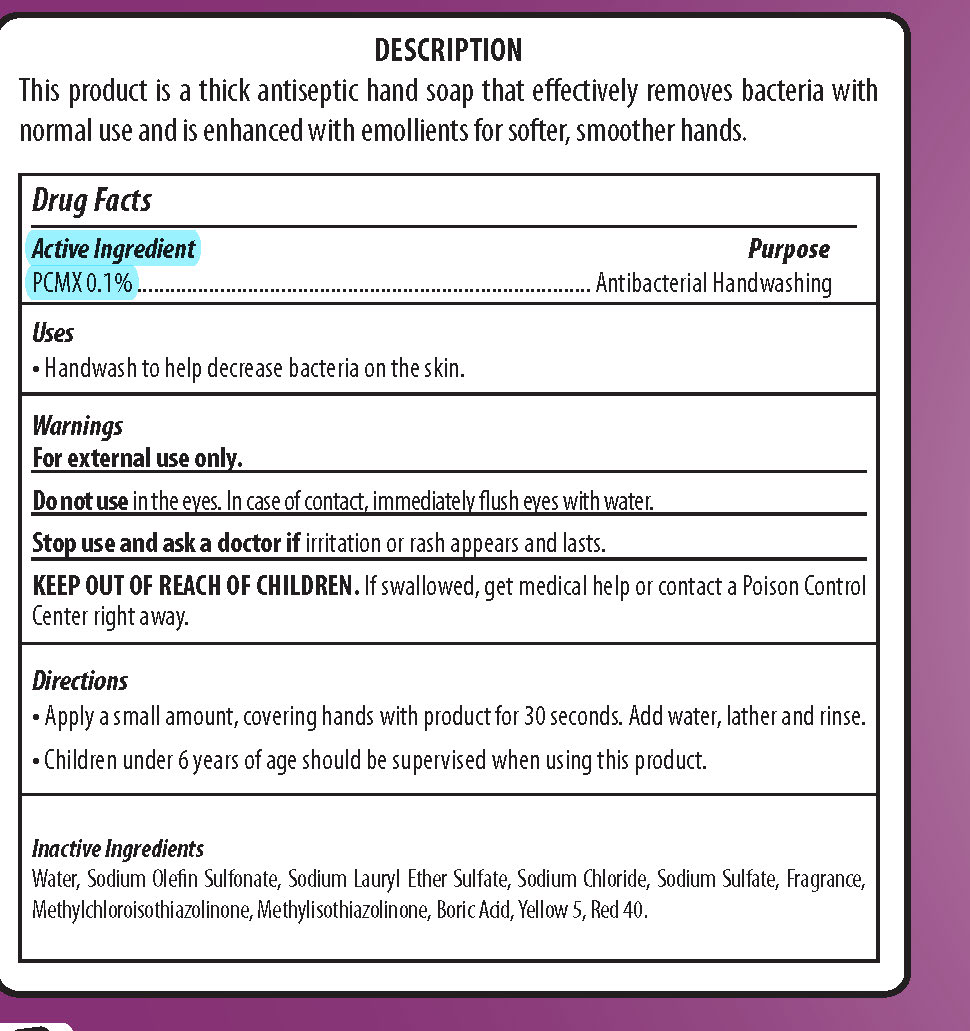
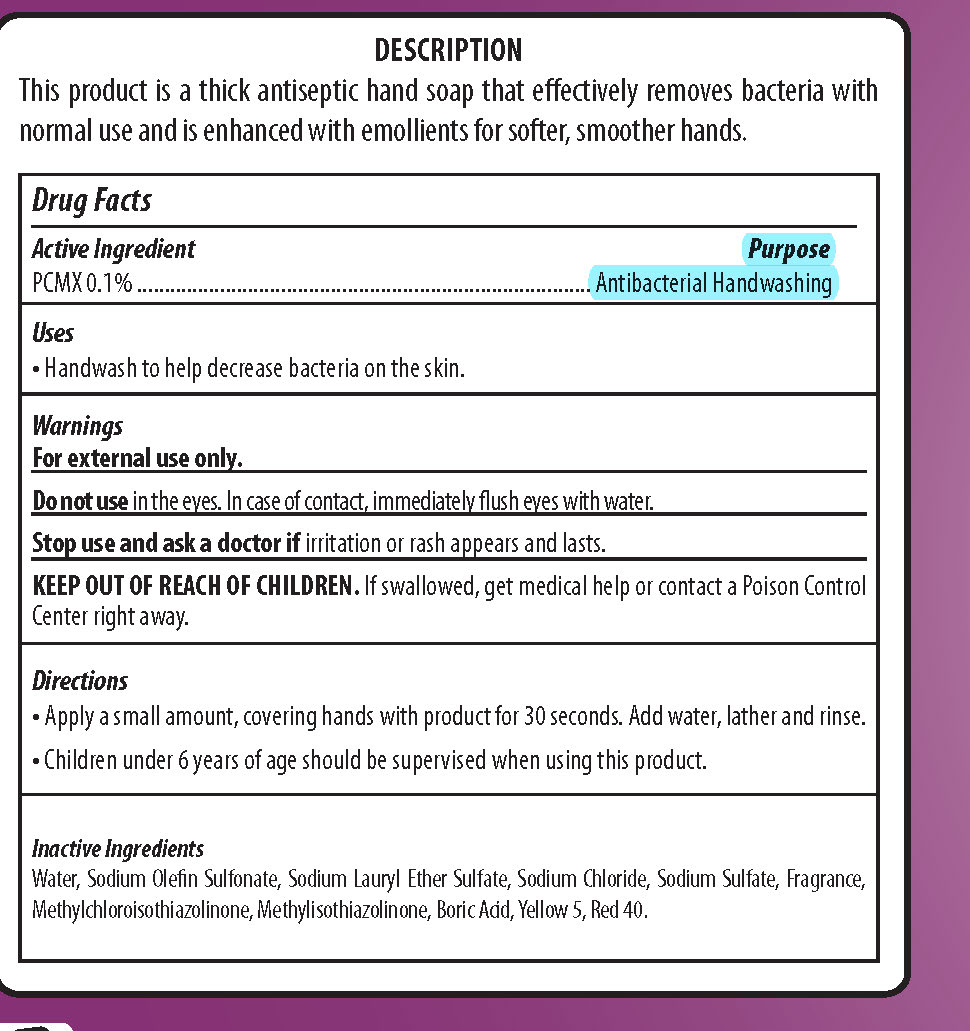
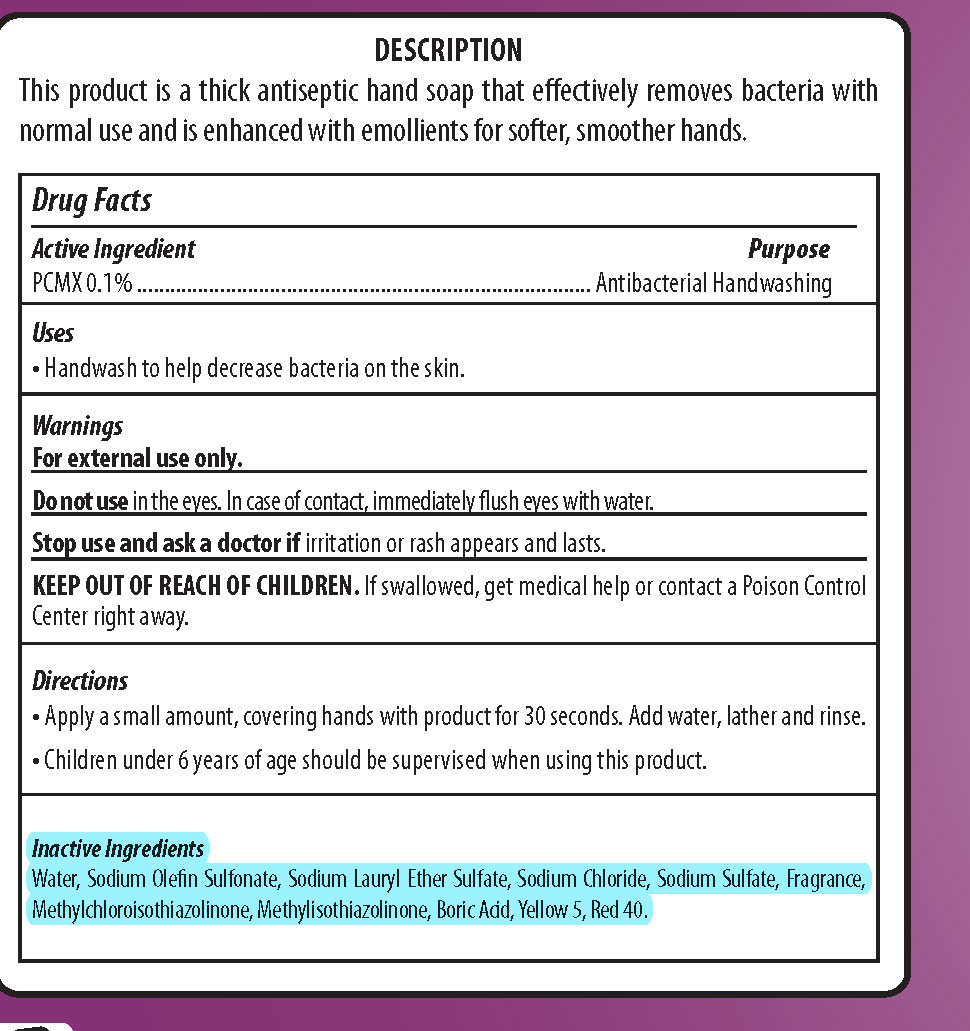
SOFT TOUCH- pcmx, dishwashing and hand soap soap
Midlab Incorporated
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
| SOFT TOUCH
pcmx, dishwashing and hand soap soap |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Midlab Incorporated (047371463) |
| Registrant - Midlab Incorporated (047371463) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Midlab Incorporated | 047371463 | manufacture(70542-704) | |
Revised: 7/2021
Document Id: c6b566da-57ca-4e06-e053-2995a90a4ee6
Set id: 562c9cab-b76f-2e11-e054-00144ff88e88
Version: 4
Effective Time: 20210709
Midlab Incorporated