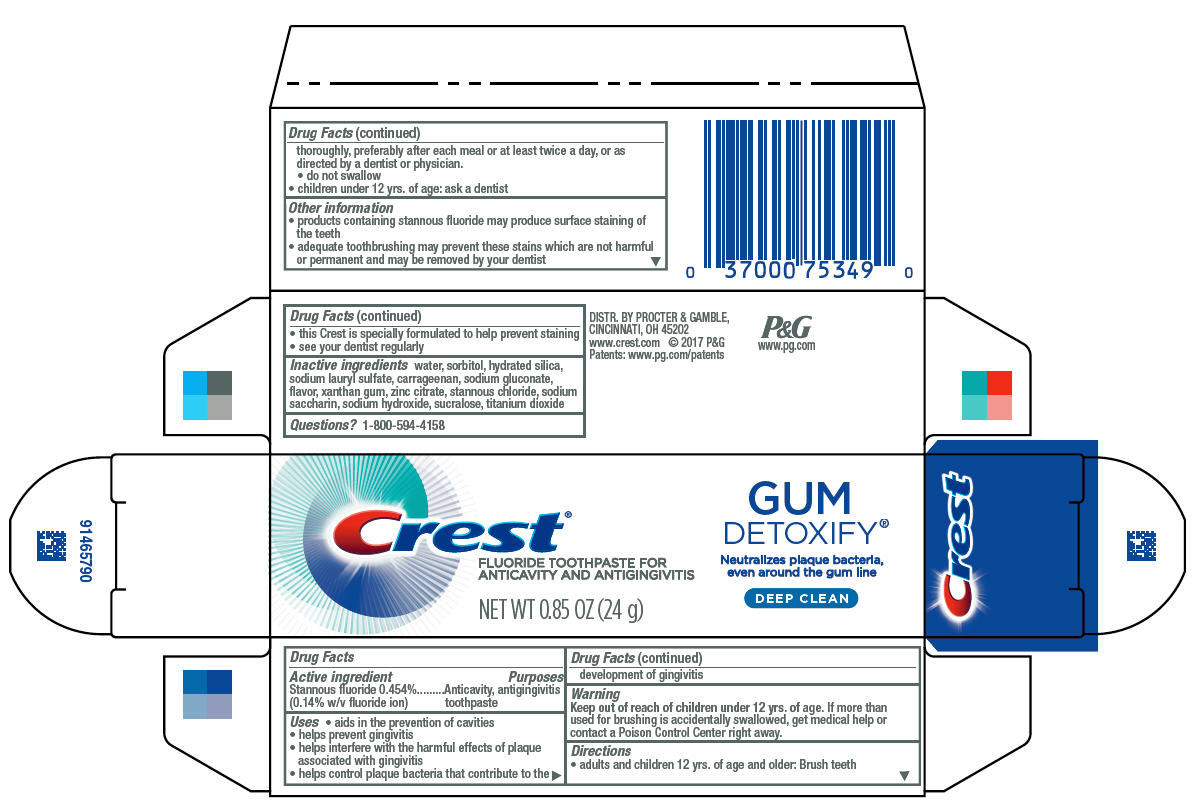
Label: CREST GUM DETOXIFY- stannous fluoride paste, dentifrice
-
NDC Code(s):
37000-481-01,
37000-481-28,
37000-481-41,
37000-481-52, view more37000-481-82
- Packager: The Procter & Gamble Manufacturing Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Purposes
- Uses
- Warning
- Directions
- KEEP OUT OF REACH OF CHILDREN
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- Principal Display Panel - 24 g tube in carton
-
INGREDIENTS AND APPEARANCE
CREST GUM DETOXIFY
stannous fluoride paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:37000-481 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength STANNOUS FLUORIDE (UNII: 3FTR44B32Q) (FLUORIDE ION - UNII:Q80VPU408O) FLUORIDE ION 1.4 mg in 1 g Inactive Ingredients Ingredient Name Strength SUCRALOSE (UNII: 96K6UQ3ZD4) STANNOUS CHLORIDE (UNII: 1BQV3749L5) WATER (UNII: 059QF0KO0R) HYDRATED SILICA (UNII: Y6O7T4G8P9) CARRAGEENAN (UNII: 5C69YCD2YJ) SODIUM GLUCONATE (UNII: R6Q3791S76) XANTHAN GUM (UNII: TTV12P4NEE) ZINC CITRATE (UNII: K72I3DEX9B) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SORBITOL (UNII: 506T60A25R) SODIUM HYDROXIDE (UNII: 55X04QC32I) SACCHARIN SODIUM (UNII: SB8ZUX40TY) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color white Score Shape Size Flavor SPEARMINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:37000-481-01 1 in 1 CARTON 08/01/2017 1 24 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:37000-481-41 1 in 1 CARTON 01/29/2018 2 116 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC:37000-481-82 2 in 1 CELLO PACK 08/01/2017 3 1 in 1 CARTON 3 116 g in 1 TUBE; Type 0: Not a Combination Product 4 NDC:37000-481-28 1 in 1 CARTON 02/03/2020 4 79 g in 1 TUBE; Type 0: Not a Combination Product 5 NDC:37000-481-52 1 in 1 CARTON 02/03/2020 5 147 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part355 08/01/2017 Labeler - The Procter & Gamble Manufacturing Company (004238200)