EYE WASH- eye wash liquid
Guangdong Comfort Medical Products Co., Ltd.
----------
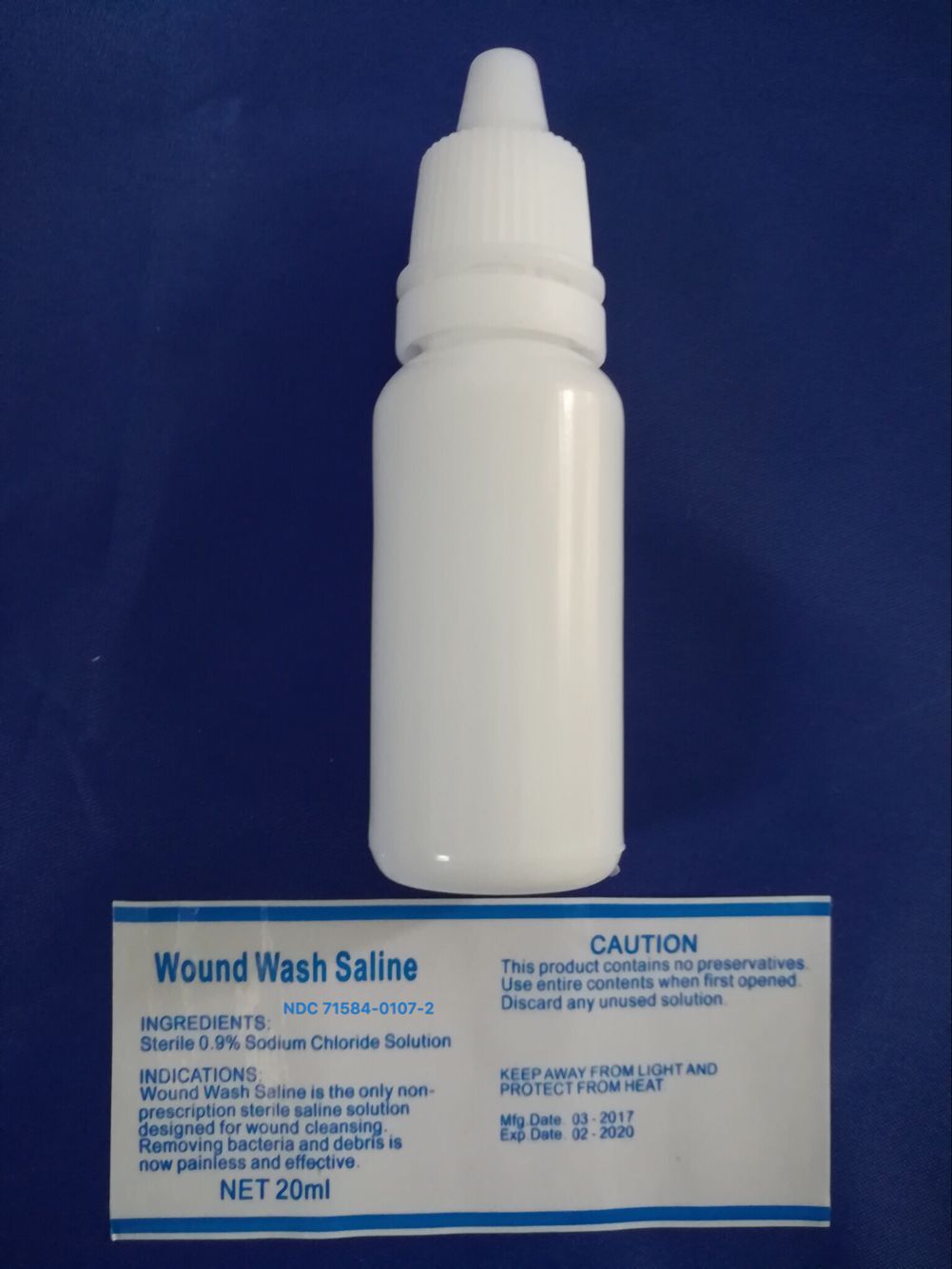
EYE WASH
- To pour - hold bottle securely, twist off top to remove
- Control rate of flow by pressure on the bottle
- Flush the affected eye(s) as needed
- Do not touch bottle tip to eye
- If necessary, continue flushing with emergency eyewash or shower.
NDC# 71584-0107-1
EYE WASH
Topical irrigation solution 0.9% w/v Sodium Chloride
For topical irrigation of eyes, burns and wounds
CAUTION:
DISCARD ANY UNUSED SOLUTION
SINGLE USE ONLY
DO NOT USE IF PACK IS DAMAGED
DO NOT RESTERILIZE AND REUSE
NOT FOR INJECTION
FOR EXTERNAL USE ONLY
STORE IN COOL DRY PLACE
KEEP OUT OF DIRECT SUNLIGHT
KEEP OUT OF REACH OF CHILDREN
20 ml
Made in China
MANUFACTURED FOR:
Total Resources Intenational, Walnut CA 91789. USA


| EYE WASH
eye wash liquid |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Guangdong Comfort Medical Products Co., Ltd. (544507534) |
| Registrant - Guangdong Comfort Medical Products Co., Ltd. (544507534) |
Revised: 3/2024
Document Id: 13d52cb9-b8bd-4aca-e063-6394a90a0f3d
Set id: 548d9fb4-bd57-74c1-e054-00144ff8d46c
Version: 8
Effective Time: 20240317
Guangdong Comfort Medical Products Co., Ltd.