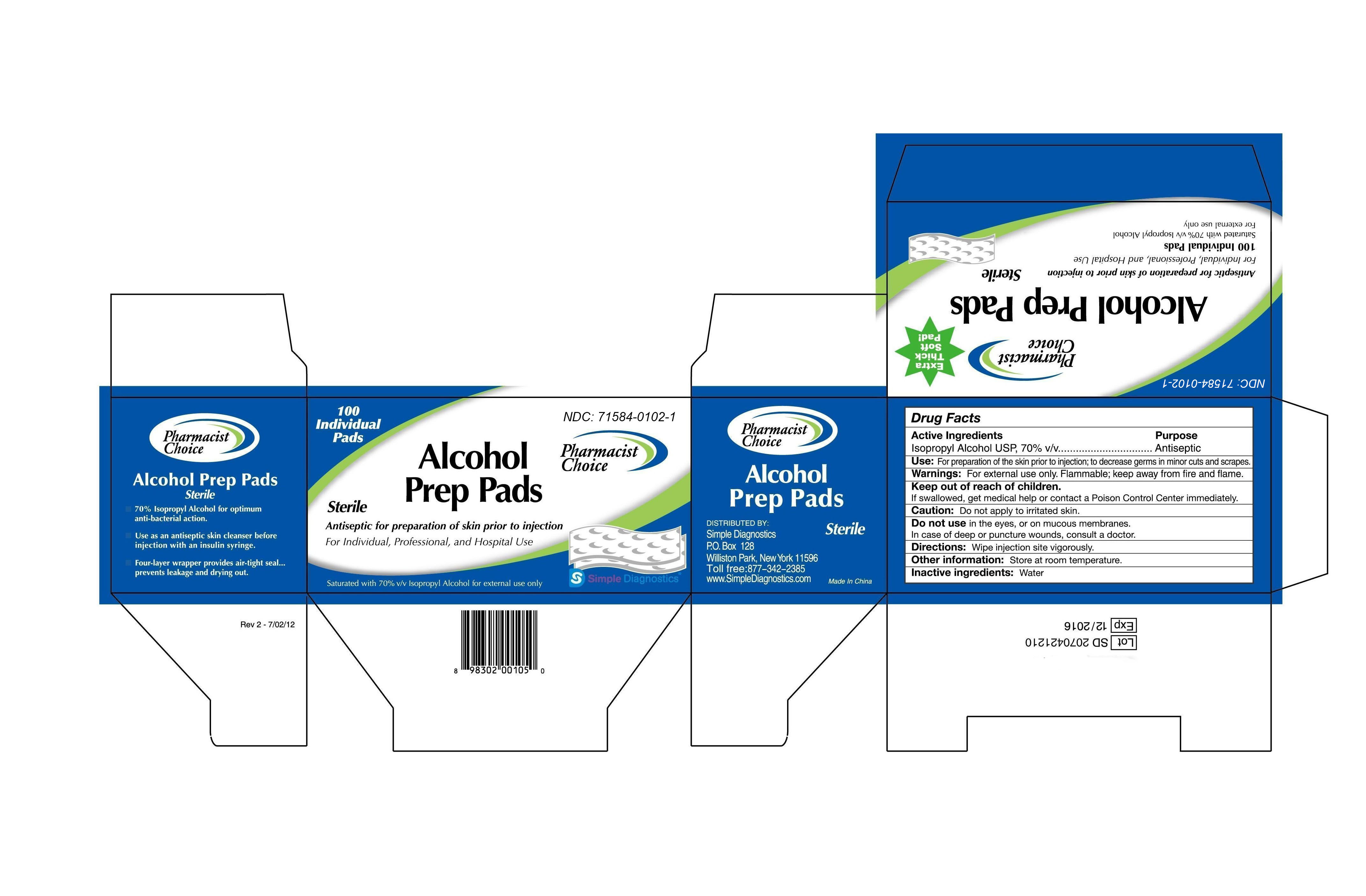
Label: PHARMACIST CHOICE ALCOHOL PREP PADS- isopropyl alcohol swab
-
Contains inactivated NDC Code(s)
NDC Code(s): 71584-0102-1 - Packager: Guangdong Comfort Medical Products Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 18, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Keep out of children
- Use
- Warning
- Directions
- Inactive ingredients
-
Drug Facts
Active Ingredients
Isopropyl Alcohol USP, 70% v/v
Purpose:
Antiseptic
Use: For preparation of skin prior to injection; to decrease germs in minor cuts and scrapes.
Warning: For external use only. Flammable, keep away from fire and flame.
Keep out of children.
If swallowed, get medical help or contact a Poison Control Center immediately.
Caution: Don’t apply to a irritated skin.
Don’t use in the eyes, or on mucous membranes.
In case of deep or puncture wounds, consult a doctor.
Directions: wipe injection site vigorously.
Other information: Store at room temperature.
Inactive ingredients: Water
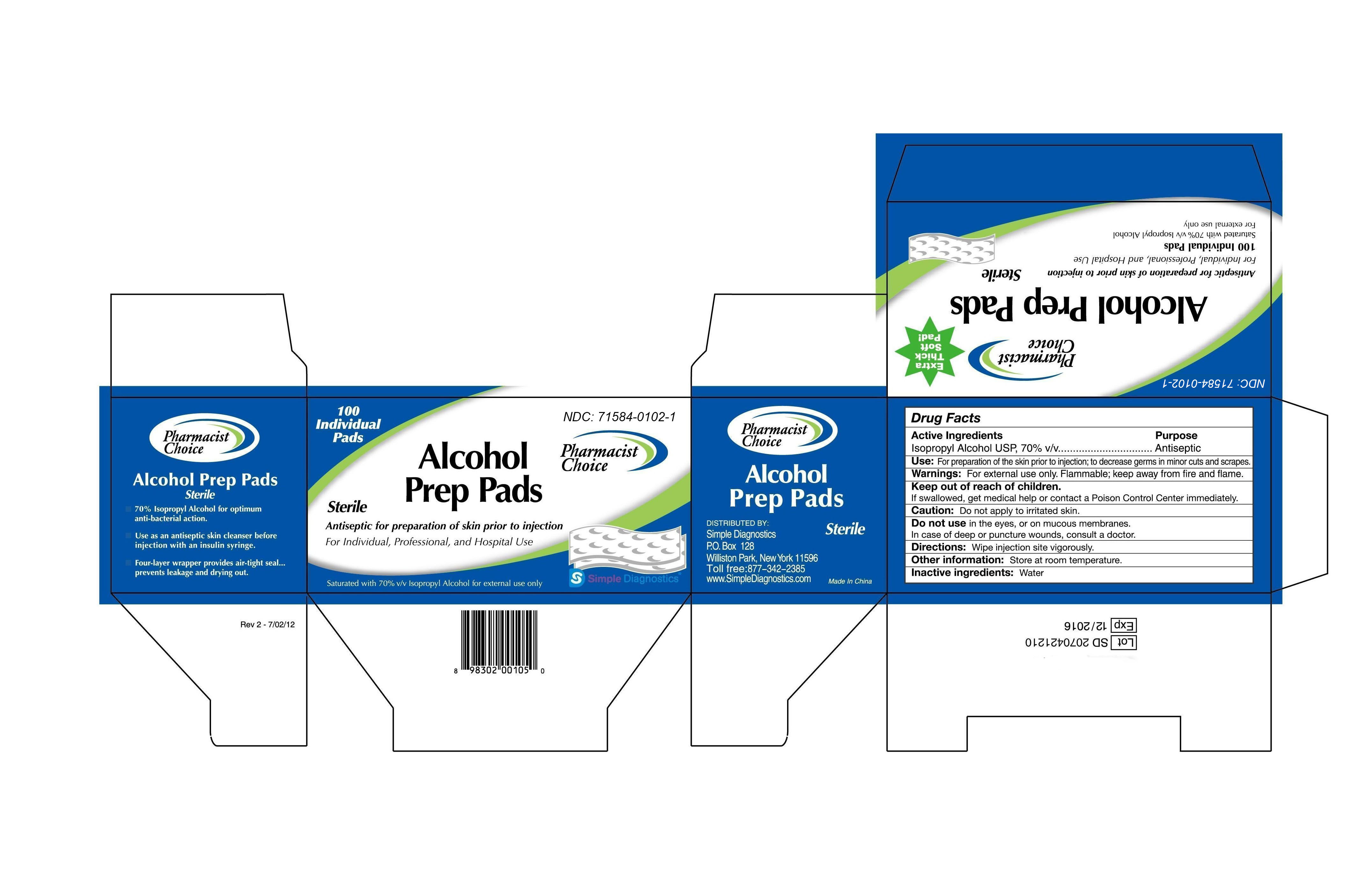
-
INGREDIENTS AND APPEARANCE
PHARMACIST CHOICE ALCOHOL PREP PADS
isopropyl alcohol swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71584-0102 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71584-0102-1 1.4 mL in 1 POUCH; Type 0: Not a Combination Product 07/17/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 07/17/2017 Labeler - Guangdong Comfort Medical Products Co., Ltd. (544507534) Registrant - Guangdong Comfort Medical Products Co., Ltd. (544507534)