NEOSPORIN ECZEMA ESSENTIALS DAILY MOISTURIZING- oatmeal cream
Johnson & Johnson Consumer Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Neosporin® Eczema Essential Daily Moisturizing Cream
Warnings
For external use only
Inactive ingredients
water, glycerin, caprylic/capric triglyceride, panthenol distearyldimonium chloride, petrolatum, isopropyl palmitate, cetyl alcohol, dimethicone, mineral oil, isopropyl myristate, ceramide NP, steareth-20, oat kernel oil, oat kernel extract, benzalkonium chloride, sodium chloride
DIST: JOHNSON & JOHNSON CONSUMER PRODUCTS COMPANY
Division of Johnson & Johnson Consumer Companies, Inc.
Skillman, NJ 08558-9418
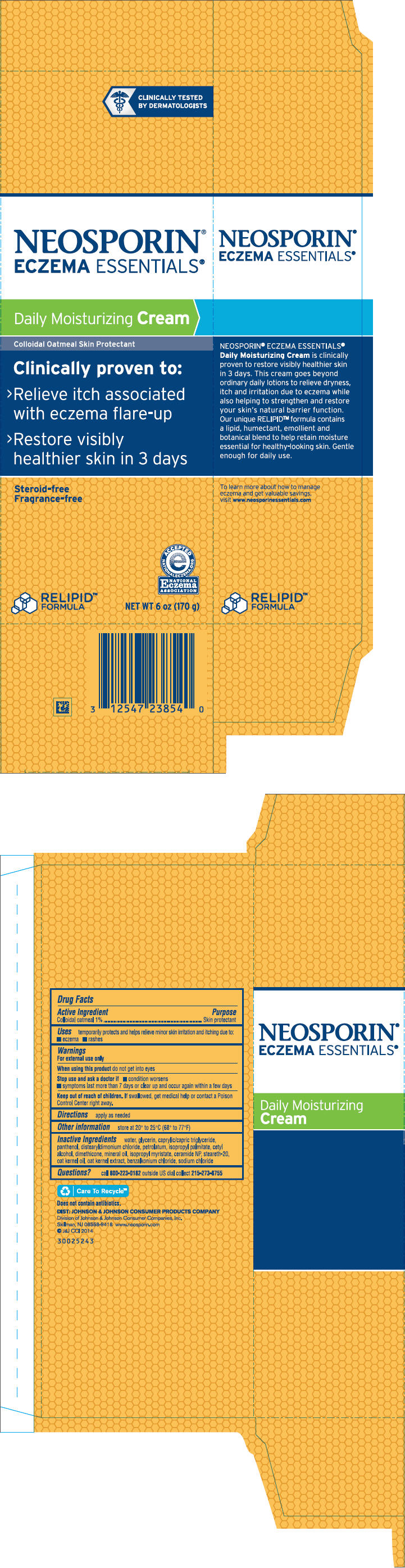
PRINCIPAL DISPLAY PANEL - 170 g Tube Carton
NEOSPORIN®
ECZEMA ESSENTIALS®
Daily Moisturizing Cream
Colloidal Oatmeal Skin Protectant
Clinically proven to:
- 〉
- Relieve itch associated
With eczema flare-up - 〉
- Restore visibly
Healthier skin in 3 days
Steroid-free
Fragrance-free
RELIPID™
FORMULA
ACCEPTED
NATIONAL ECZEMA.ORG
NATIONAL
ECZEMA
ASSOCIATION
NET WT 6 oz (170 g)
| NEOSPORIN ECZEMA ESSENTIALS
DAILY MOISTURIZING
oatmeal cream |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Johnson & Johnson Consumer Inc. (002347102) |