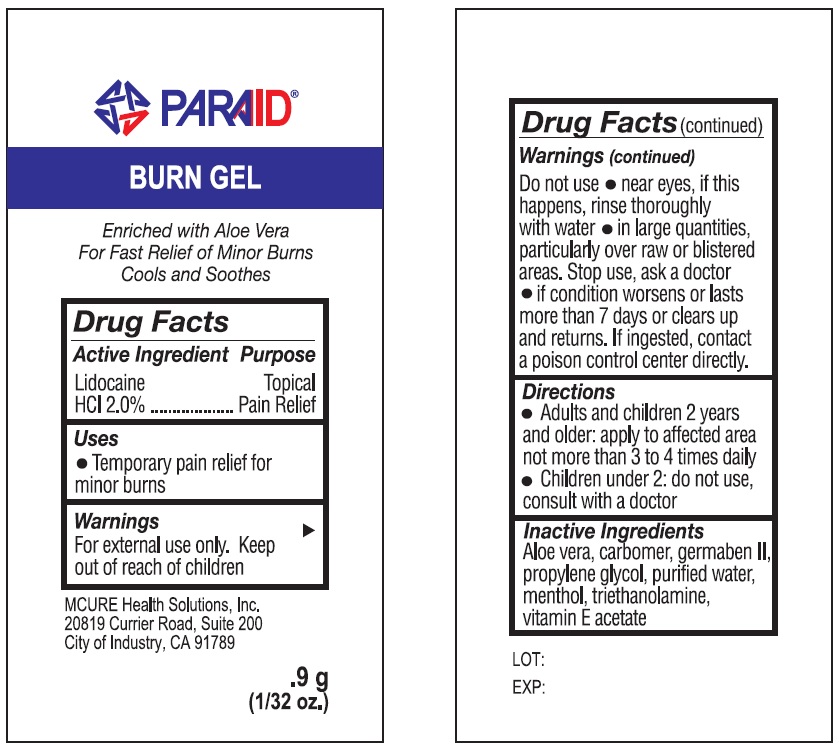
PARAID BURN- lidocaine hydrochloride gel
Mcure Health Solutions Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Paraid Burn Gel
Warnings
For external use only.
Directions
- Adults and children 2 years and older: apply to affected area not more than 3 to 4 times daily
- Children under 2: do not use, consult with a doctor
| PARAID BURN
lidocaine hydrochloride gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Mcure Health Solutions Inc. (053034873) |
Revised: 5/2017
Document Id: 4e781310-4fbb-2c49-e054-00144ff8d46c
Set id: 54404754-fc6b-4e07-b399-f9091ab22f1a
Version: 3
Effective Time: 20170501
Mcure Health Solutions Inc.