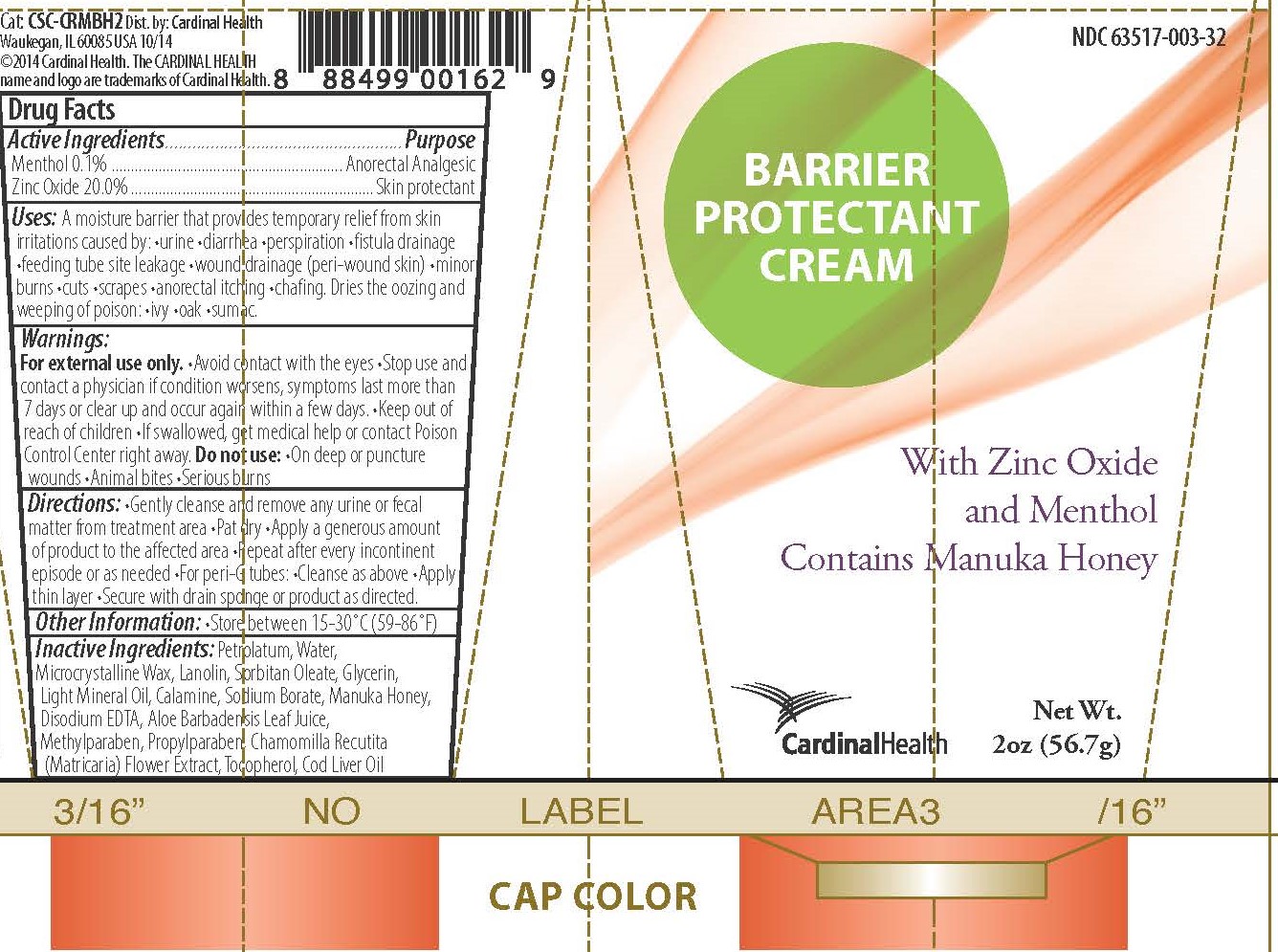
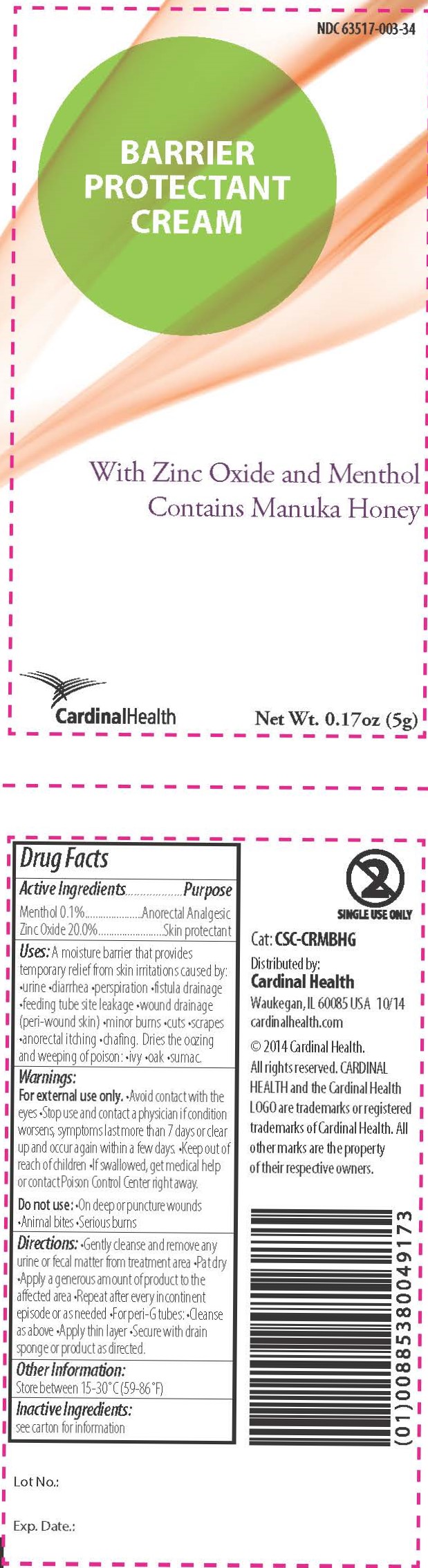
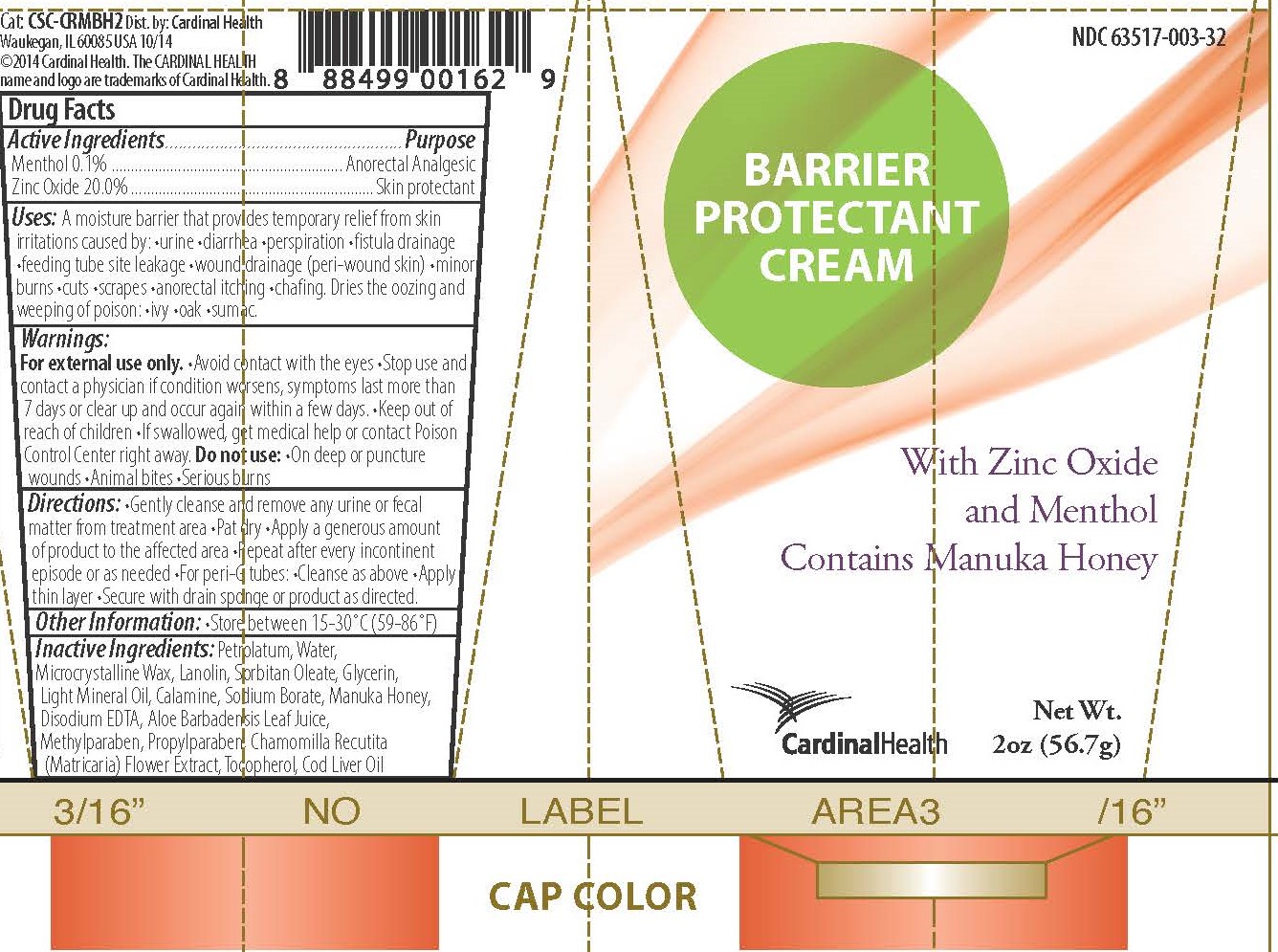
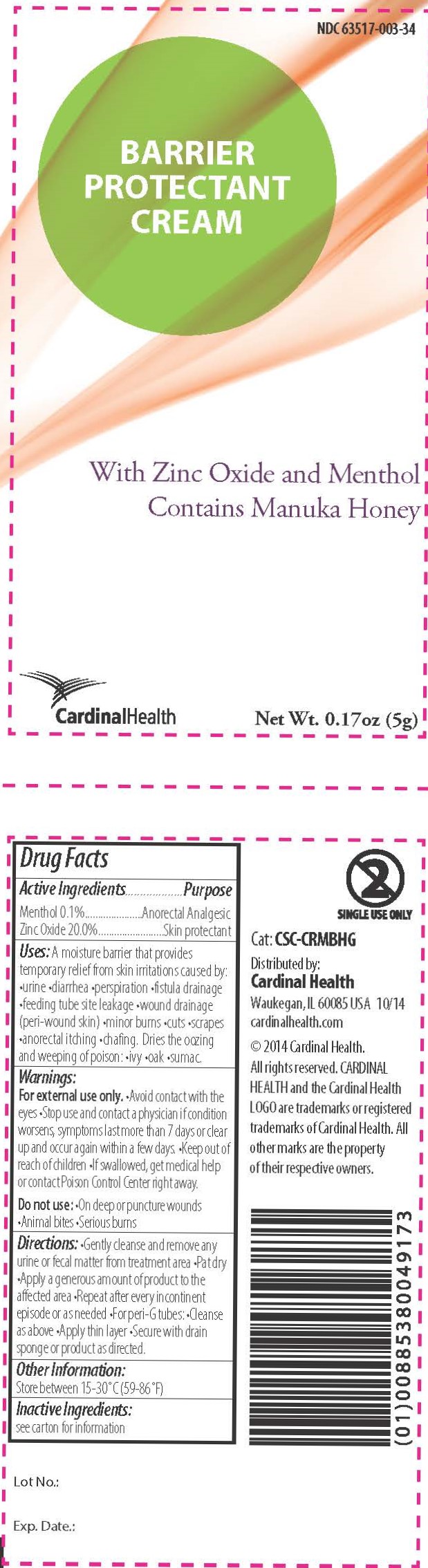
Label: BARRIER PROTECTANT CREAM- barrier cream ointment
-
Contains inactivated NDC Code(s)
NDC Code(s): 63517-003-31, 63517-003-32, 63517-003-34 - Packager: Cardinal Health
- This is a repackaged label.
- Source NDC Code(s): 51028-002
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 26, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
-
Uses
A moisture barrier that provides temporary relief from skin irritations caused by •urine •diarrhea •perspiration •fistula drainage •feeding tube site leakage •wound drainage (peri-wound skin) •minor burns •cuts •scrapes •anorectal itching •chafing
Dries the oozing and weeping of poison •ivy •oak •sumac
-
Warnings
For external use only.
- Avoid contact with the eyes
- Stop use and contact a physician if condition worsens, symptoms last more than 7 days, or clear up and occur again within a few days.
- Keep out of reach of children
- If swallowed, get medical help or contact Poison Control Center right away.
Do not use:
- On deep or puncture wounds
- Animal bites
- Serious burns
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive Ingredients
- Barrier Protectant Cream Labeling
-
INGREDIENTS AND APPEARANCE
BARRIER PROTECTANT CREAM
barrier cream ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63517-003(NDC:51028-002) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1 mg in 1 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 200 mg in 1 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) FERRIC OXIDE RED (UNII: 1K09F3G675) CHAMOMILE (UNII: FGL3685T2X) COD LIVER OIL (UNII: BBL281NWFG) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) LANOLIN (UNII: 7EV65EAW6H) LIGHT MINERAL OIL (UNII: N6K5787QVP) HONEY (UNII: Y9H1V576FH) METHYLPARABEN (UNII: A2I8C7HI9T) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) PETROLATUM (UNII: 4T6H12BN9U) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM BORATE (UNII: 91MBZ8H3QO) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) TOCOPHEROL (UNII: R0ZB2556P8) WATER (UNII: 059QF0KO0R) Product Characteristics Color pink Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63517-003-31 113 g in 1 TUBE; Type 0: Not a Combination Product 01/15/2015 2 NDC:63517-003-32 57 g in 1 TUBE; Type 0: Not a Combination Product 01/15/2015 3 NDC:63517-003-34 150 in 1 BOX 01/15/2015 3 5 g in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part346 01/15/2015 Labeler - Cardinal Health (961027315)