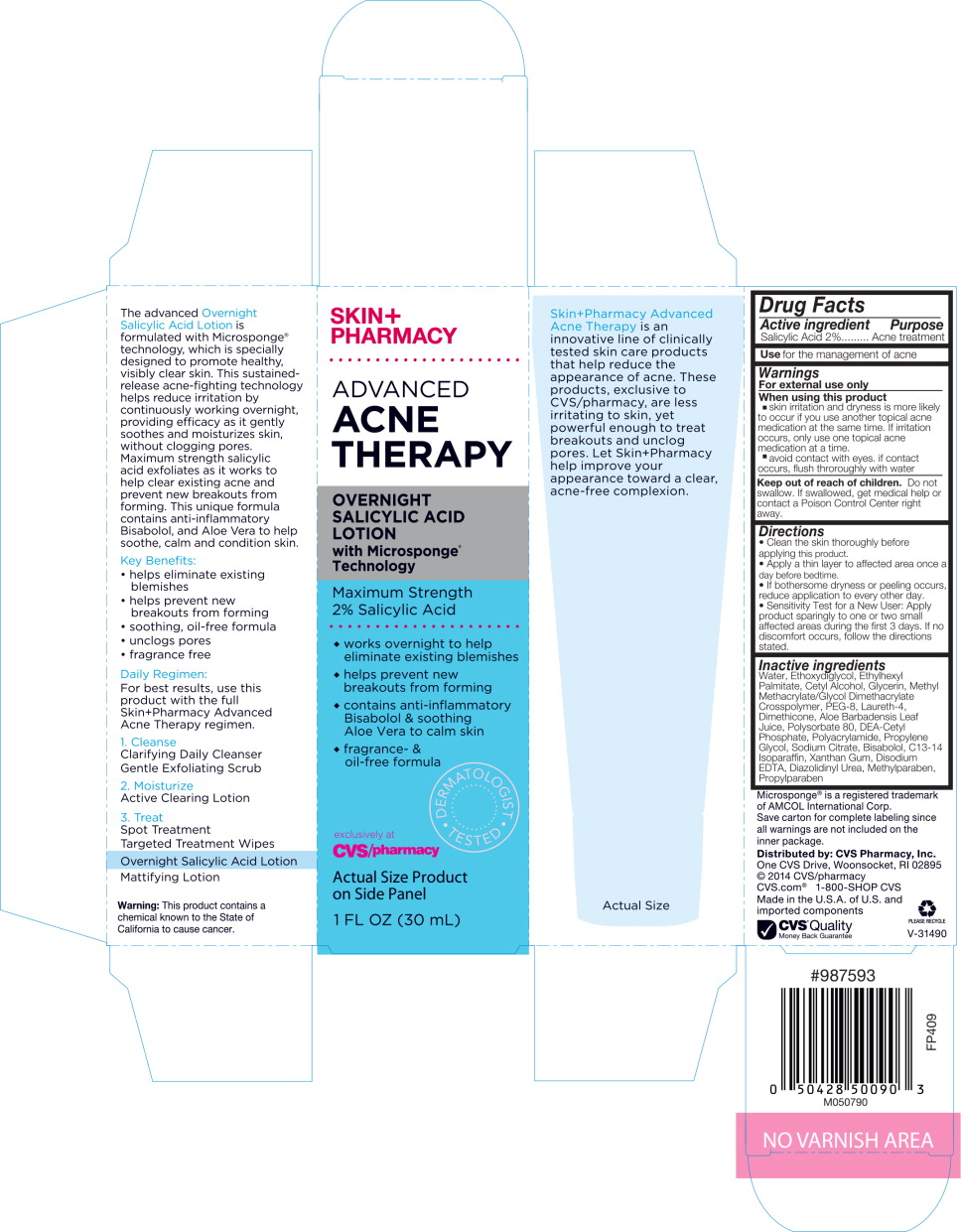
Label: SKINPHARMACY ADVANCED ACNE THERAPY OVERNIGHT SALICYLIC ACID- salicylic acid liquid
- NDC Code(s): 69842-066-01
- Packager: CVS Health
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 14, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
-
Warnings
For external use only
-
Directions
- Clean the skin thoroughly before applying this product.
- Apply a thin layer to affected area once a day before bedtime.
- If bothersome dryness or peeling occurs, reduce application to every other day.
- Sensitivity Test for a New User: Apply product sparingly to one or two small affected areas during the first 3 days. If no discomfort occurs, follow the directions stated.
-
Inactive ingredients
Water, Ethoxydiglycol, Ethylhexyl Palmitate, Cetyl Alcohol, Glycerin, Methyl Methacrylate/Glycol Dimethacrylate Crosspolymer, PEG-8, Laureth-4, Dimethicone, Aloe Barbadensis Leaf Juice, Polysorbate 80, DEA-Cetyl Phosphate, Polyacrylamide, Propylene Glycol, Sodium Citrate, Bisabolol, C13-14 Isoparaffin, Xanthan Gum, Disodium EDTA, Diazolidinyl Urea, Methylparaben, Propylparaben
Microsponge® is a registered trademark of AMCOL International Corp.
Save carton for complete labeling since all warnings are not included on the inner package.
Distributed by: CVS Pharmacy, Inc.
One CVS Drive, Woonsocket, RI 02895
© 2014 CVS/pharmacy
CVS.com® 1-800-SHOP CVS
Made in the U.S.A. of U.S. and foreign components
CVS® Quality
Money Back Guarantee
V-13649
PLEASE RECYCLE
V-31490
#987593
FP409
M050790
-
Principal Display Panel - Carbon Label
SKIN+
PHARMACYADVANCED
ACNE
THERAPYOVERNIGHT
SALICYLIC
ACID
LOTION
with Microsponge®
TechnologyMaximum Strength 2% Salicylic Acid
- works overnight to help eliminate existing blemishes
- helps prevent new breakouts from forming
- contains anti-inflammatory Bisabolol & soothing Ale Vera to calm skin
- fragrance- & oil-free formula
DERMATOLOGIST TESTED
exclusively at
CVS/pharmacyActual Size Product
on Side Panel1 FL OZ (30 mL)
-
Principal Display Panel - Tube Label
SKIN+PHARMACY
ADVANCED
ACNE
THERAPYOVERNIGHT
SALICYLIC ACID LOTION
with Microsponge® TechnologyMaximum Strength
2% Salicylic Acid- works overnight to help eliminate existing blemishes
- helps prevent new breakouts from forming
- contains anti-inflammatory Bisabolol & soothing Aloe Vera to calm skin
- fragrance- & oil-free formula
DERMATOLOGIST TESTED
exclusively at
CVS/pharmacy1 FL OZ (30 mL)
-
INGREDIENTS AND APPEARANCE
SKINPHARMACY ADVANCED ACNE THERAPY OVERNIGHT SALICYLIC ACID
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69842-066 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength salicylic acid (UNII: O414PZ4LPZ) (salicylic acid - UNII:O414PZ4LPZ) salicylic acid 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) ETHYLHEXYL PALMITATE (UNII: 2865993309) CETYL ALCOHOL (UNII: 936JST6JCN) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) LAURETH-4 (UNII: 6HQ855798J) DIMETHICONE (UNII: 92RU3N3Y1O) ALOE VERA LEAF (UNII: ZY81Z83H0X) POLYSORBATE 80 (UNII: 6OZP39ZG8H) DIETHANOLAMINE CETYL PHOSPHATE (UNII: 4UG0316V9S) POLYACRYLAMIDE (1500 MW) (UNII: 5D6TC4BRWV) 1,2-PROPANEDIOL, 1-BENZOATE (UNII: K4K90ZQ89N) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) LEVOMENOL (UNII: 24WE03BX2T) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) XANTHAN GUM (UNII: TTV12P4NEE) EDETATE DISODIUM (UNII: 7FLD91C86K) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69842-066-01 1 in 1 CARTON 05/01/2014 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 05/01/2014 Labeler - CVS Health (062312574) Registrant - AMCOL Health & Beauty Solutions, Inc. DBA (872684803) Establishment Name Address ID/FEI Business Operations AMCOL Health & Beauty Solutions, Inc. DBA 872684803 ANALYSIS(69842-066) , MANUFACTURE(69842-066) , LABEL(69842-066) , PACK(69842-066)