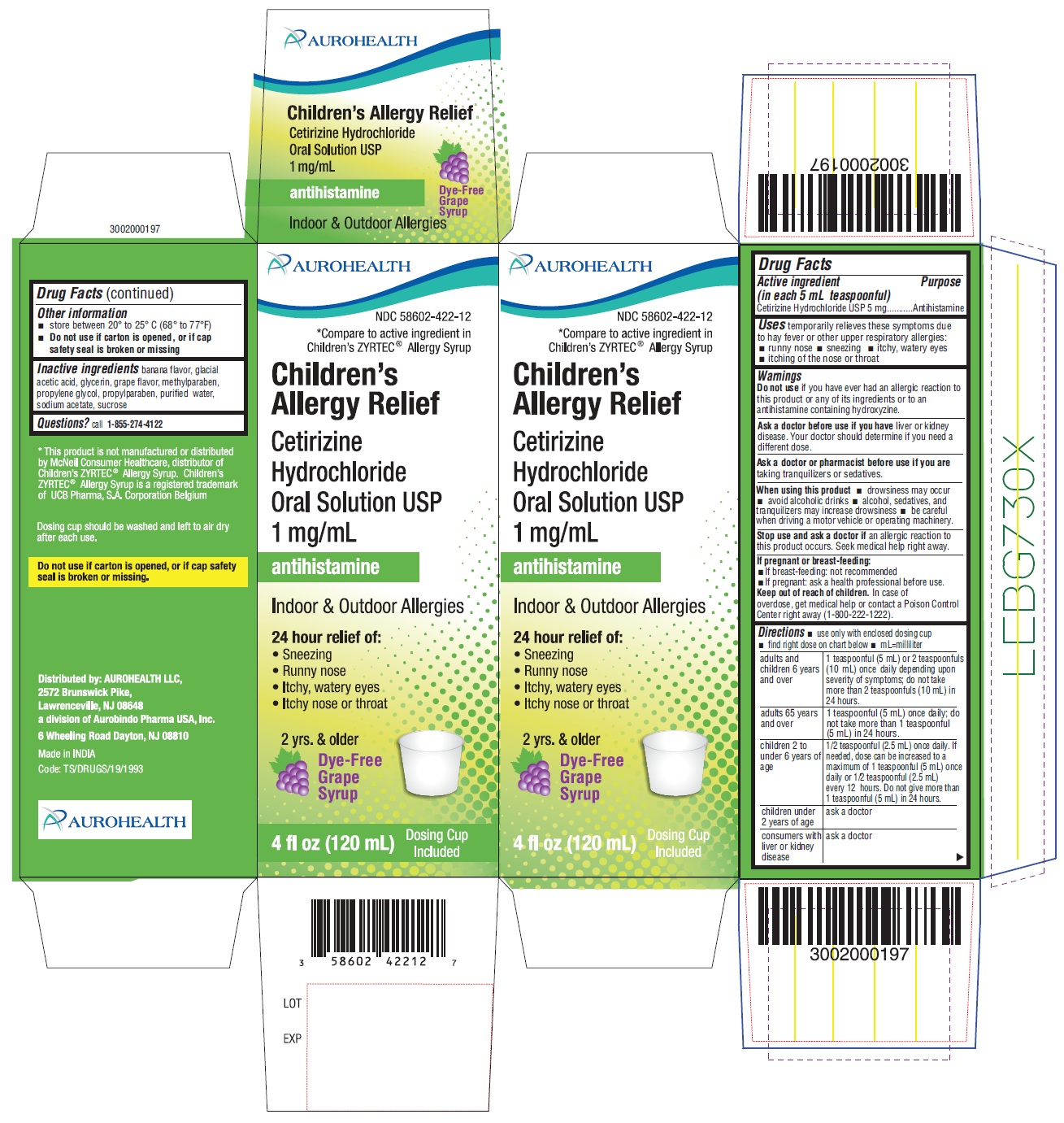
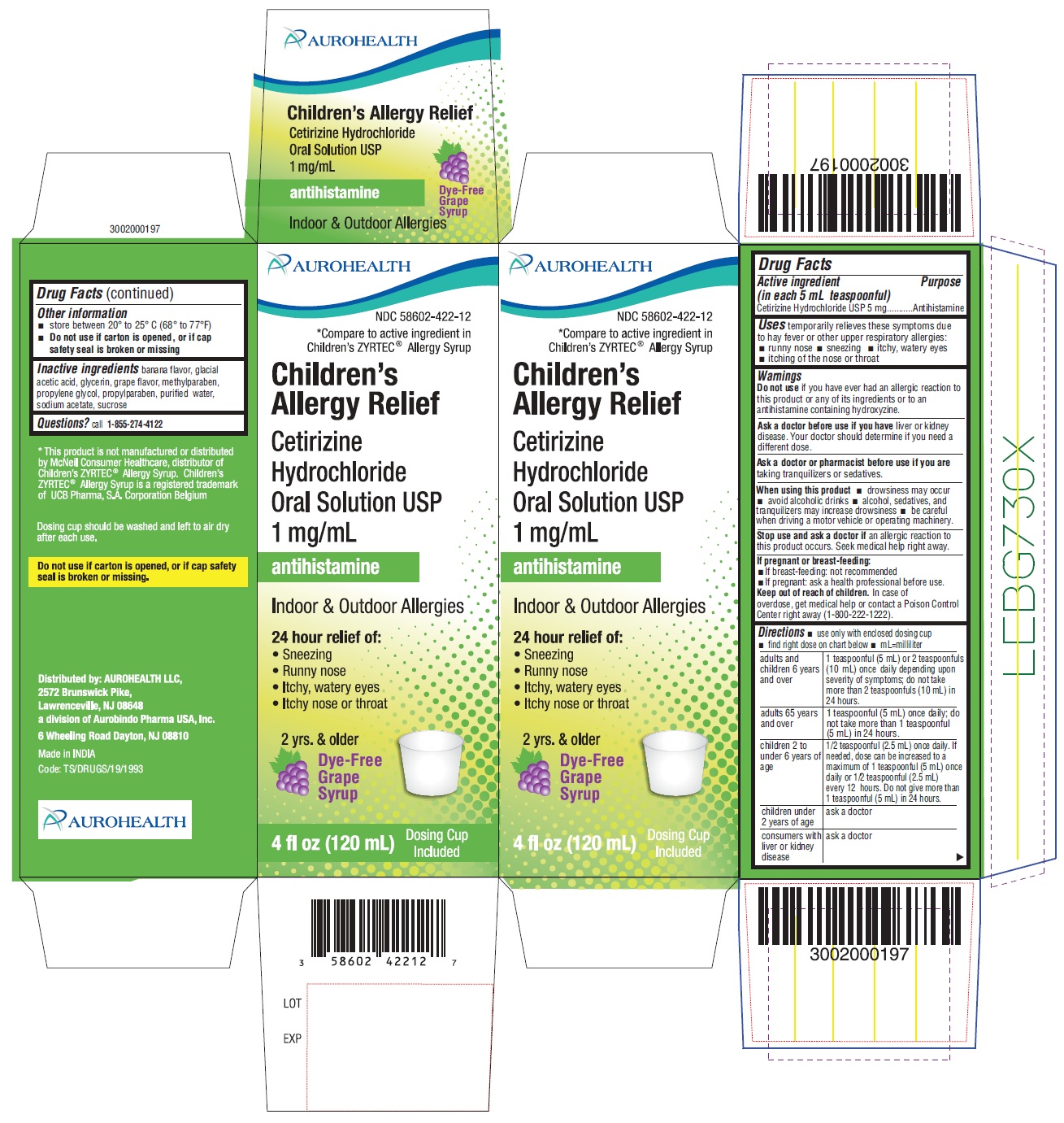
Label: CHILDRENS ALLERGY RELIEF- cetirizine hydrochloride solution
- NDC Code(s): 58602-422-12, 58602-422-20
- Packager: Aurohealth LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 3, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Uses
-
Warnings
Do not use
if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
Ask a doctor before use if you have
liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery.
Stop use and ask a doctor if
an allergic reaction to this product occurs. Seek medical help right away.
-
Directions
- use only with enclosed dosing cup
- find right dose on chart below
- mL=milliliter
adults and children 6 years and over
1 teaspoonful (5 mL) or 2 teaspoonfuls (10 mL) once daily depending upon severity of symptoms; do not take more than 2 teaspoonfuls (10 mL) in 24 hours.
adults 65 years and over
1 teaspoonful (5 mL) once daily; do not take more than 1 teaspoonful (5 mL) in 24 hours.
children 2 to under 6 years of age
½ teaspoonful (2.5 mL) once daily. If needed, dose can be increased to a maximum of 1 teaspoonful (5 mL) once daily or ½ teaspoonful (2.5 mL) every 12 hours. Do not give more than 1 teaspoonful (5 mL) in 24 hours.
children under 2 years of age
ask a doctor
consumers with liver or kidney disease
ask a doctor
- Other information
- Inactive ingredients
-
Questions?
call 1-855-274-4122
*This product is not manufactured or distributed by McNeil Consumer Healthcare, distributor of Children's ZYRTEC® Allergy Syrup. Children's ZYRTEC® Allergy Syrup is a registered trademark of UCB Pharma, S.A. Corporation Belgium
Dosing cup should be washed and left to air dry after each use.
Do not use if carton is opened, or if cap safety seal is broken or missing.
Distributed by: AUROHEALTH LLC,
2572 Brunswick Pike,
Lawrenceville, NJ 08648
a division of Aurobindo Pharma USA, Inc.
6 Wheeling Road, Dayton NJ 08810
Made in INDIA
Code: TS/DRUGS/19/1993 -
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1 mg/mL (120 mL Bottle)
AUROHEALTH
NDC 58602-422-12
*Compare to active ingredient in Children's ZYRTEC®Allergy Syrup
Children's Allergy Relief
Cetirizine Hydrochloride Oral Solution USP 1 mg/mL
antihistamine
Indoor & Outdoor Allergies
24 hour relief of:- Sneezing
- Runny nose
- Itchy, watery eyes
- Itchy nose or throat
Dye Free Grape Syrup
4 fl oz (120 mL) 2 yrs. & older

-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1 mg/mL Carton (120 mL)
AUROHEALTH
NDC 58602-422-12
*Compare to active ingredient in Children’s ZYRTEC® Allergy Syrup
Children's Allergy Relief
Cetirizine Hydrochloride Oral Solution USP
1 mg/mL
antihistamine
Indoor & Outdoor Allergies
24 hour Relief of- Sneezing
- Runny nose
- Itchy, watery eyes
- Itchy nose or throat
2 yrs. & older
Dye-Free Grape Syrup
4 fl oz (120 mL) Dosing Cup Included
-
INGREDIENTS AND APPEARANCE
CHILDRENS ALLERGY RELIEF
cetirizine hydrochloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58602-422 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength ACETIC ACID (UNII: Q40Q9N063P) GLYCERIN (UNII: PDC6A3C0OX) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SODIUM ACETATE (UNII: 4550K0SC9B) SUCROSE (UNII: C151H8M554) Product Characteristics Color YELLOW (Colorless to Pale Yellow) Score Shape Size Flavor BANANA, GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58602-422-12 1 in 1 CARTON 02/02/2010 1 120 mL in 1 BOTTLE; Type 1: Convenience Kit of Co-Package 2 NDC:58602-422-20 1 in 1 CARTON 02/02/2010 2 240 mL in 1 BOTTLE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090750 02/02/2010 Labeler - Aurohealth LLC (078728447) Establishment Name Address ID/FEI Business Operations Aurobindo Pharma Limited 918917642 ANALYSIS(58602-422) , MANUFACTURE(58602-422)