Label: GOODSENSE SENNA- sennosides tablet
-
Contains inactivated NDC Code(s)
NDC Code(s): 50804-180-01 - Packager: Geiss, Destin & Dunn, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 19, 2012
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
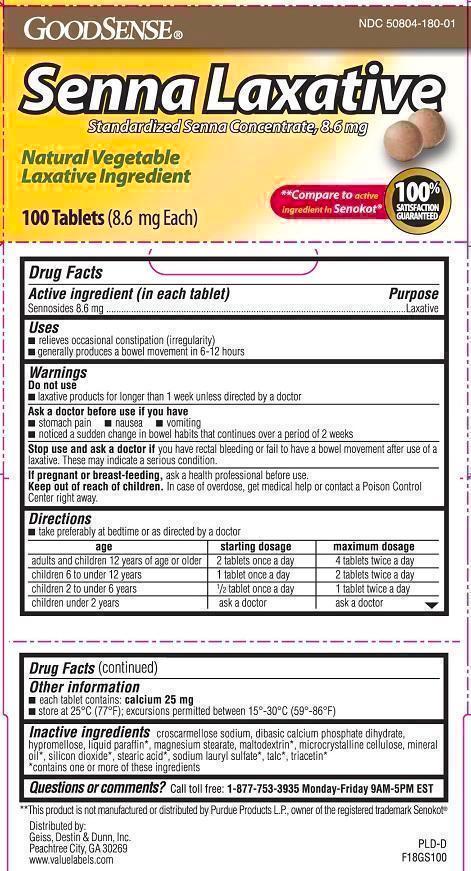
- Active Ingredient
- Purpose
- Uses:
-
WARNINGS
Do not use Laxative products for longer than 1 week, unless directed by a doctor
Ask a doctor befor use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that continues over a period of 2 weeks
Stop use and ask a doctor
if you have rectal bleeding or fail to have a bowel movement after use of a laxative. These may indicate a serious condition.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Other Information
- each tablet contains: calcium 25 mg/tablet
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- do not use if imprinted safety seal under cap is broken or missing
- This product is not manufactured or distributed by Purdue Products L.P., owner of the registered trademark Senokot®
-
Directions
Take preferably at bedtime or as directed by a doctor.
Age
Starting Dosage
Maximum Dosage
adults and children 12 years of age or older
2tablets once a day
4 tablets twice a day
children 6 to under 6 years
1 tablet once a day
2 tablets twice a day
children 2 to under 6 years
1/2 tablet once a day
1 tablet twice a day
children under 2 years
ask a doctor
ask a doctor
- Inactive Ingredients
- Other Information
- Questions or Comments?
- Product Labeling
-
INGREDIENTS AND APPEARANCE
GOODSENSE SENNA
sennosides tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50804-180 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNOSIDES A AND B (UNII: 1B5FPI42EN) (SENNOSIDES A AND B - UNII:1B5FPI42EN) SENNOSIDES A AND B 8.6 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) CALCIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: O7TSZ97GEP) HYPROMELLOSES (UNII: 3NXW29V3WO) PARAFFIN (UNII: I9O0E3H2ZE) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MINERAL OIL (UNII: T5L8T28FGP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TALC (UNII: 7SEV7J4R1U) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color brown Score no score Shape ROUND Size 9mm Flavor Imprint Code S3;S8;TCL080 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50804-180-01 1 in 1 BOX 1 100 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part334 11/02/2012 Labeler - Geiss, Destin & Dunn, Inc. (076059836)