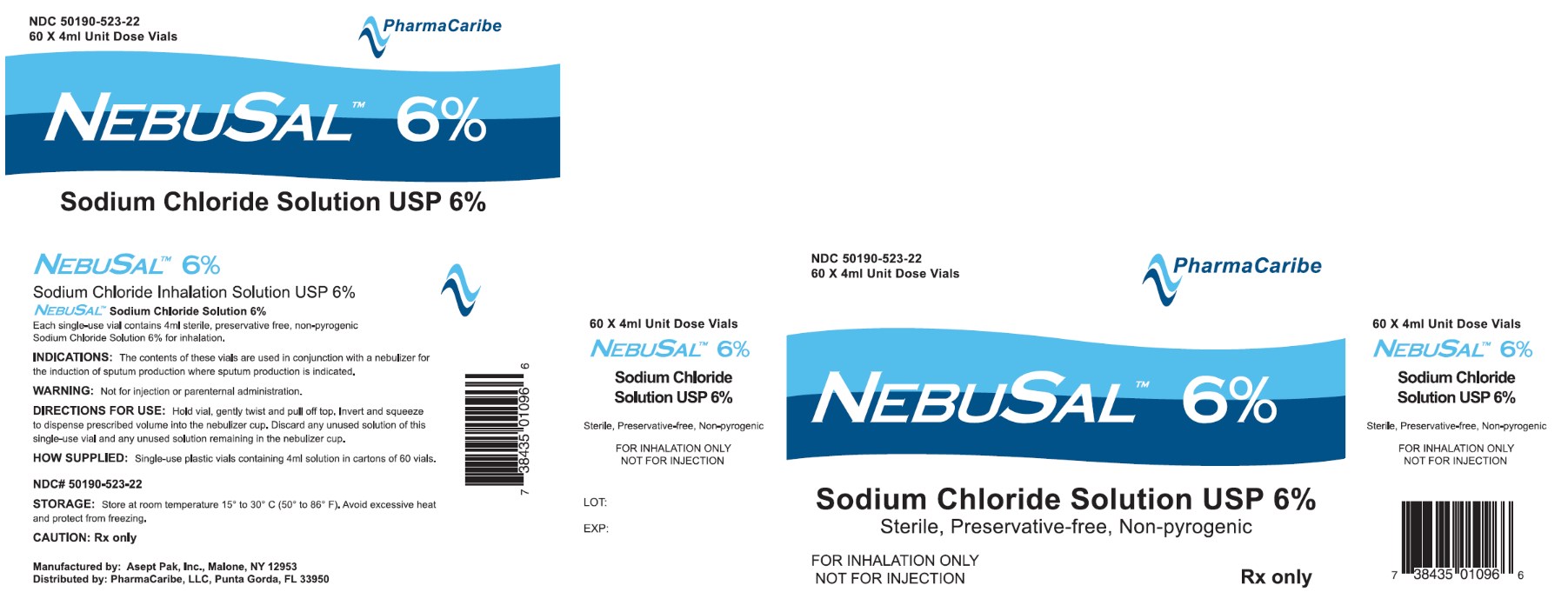
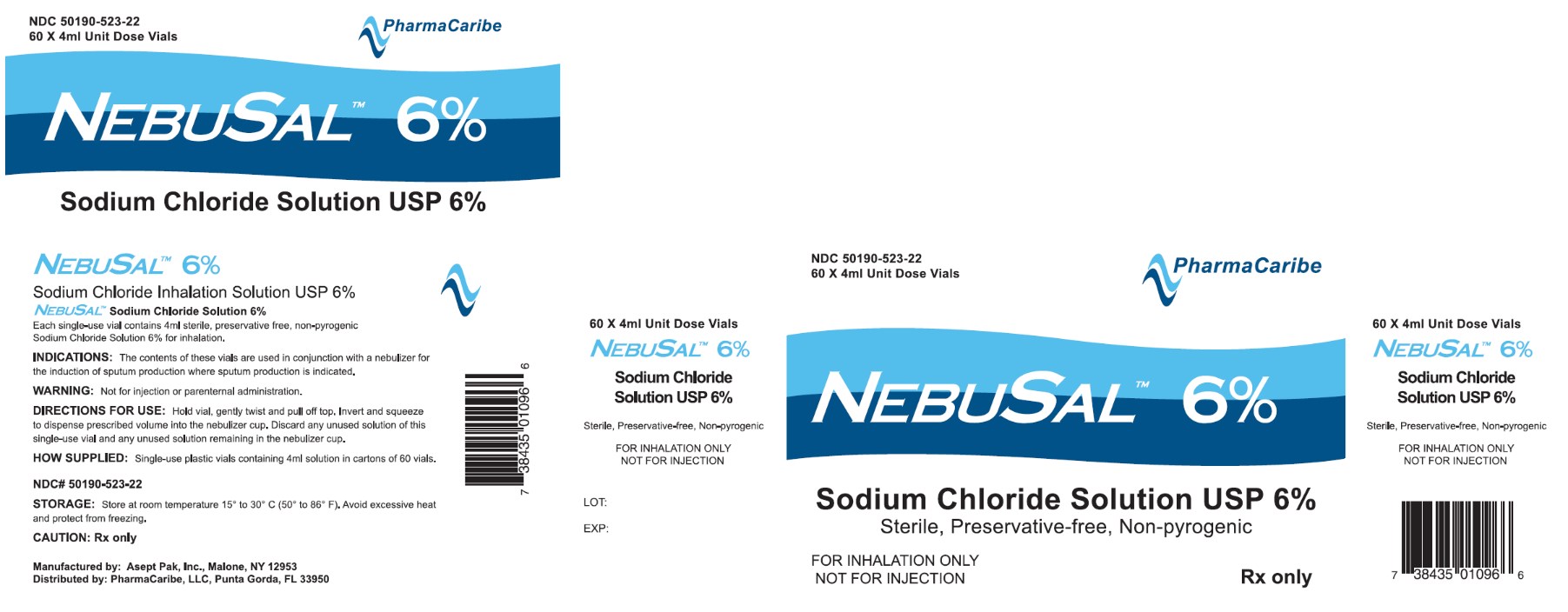
Label: NEBUSAL-
- NHRIC Code(s): 50190-523-22
- Packager: PharmaCaribe
- Category: MEDICAL DEVICE
- DEA Schedule: None
- Marketing Status: Exempt device
Drug Label Information
Updated September 12, 2013
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Sodium Chloride Inhalation Solution USP 6% 4 mL
For Respiratory Therapy
Not for parenteral administration.
4 mL, 60 Vials Sterile Unit-Dose Vial
Discard any unused portion of the contents of this single-use vial as well as any unused solution remaining in the nebulizer cup.
Internal contents sterile. External surface of vial not sterile.
- INDICATIONS AND USAGE
- INSTRUCTIONS FOR USE SECTION
- How Supplied
- WARNINGS
- STORAGE
- Sodium Chloride Inhalation Solution USP 6% 4 mL
-
INGREDIENTS AND APPEARANCE
NEBUSAL
nebulizer (direct patient interface)Product Information Product Type MEDICAL DEVICE Item Code (Source) NHRIC:50190-523 Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 240 mg in 4 mL Product Characteristics number of times usable 1 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:50190-523-22 60 in 1 BOX 1 4 mL in 1 VIAL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date exempt device CAF 08/17/2010 Labeler - PharmaCaribe (011702541) Registrant - PharmaCaribe (011702541) Establishment Name Address ID/FEI Business Operations PharmaCaribe 011702541 label