PL-0591- flexi hand sanitizer gel
Broder Bros Co.,
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Warnings
For external use only.
Reference: PL-0591 Artwork -Back.jpg
Warnings
Do not use in the eyes. In case of contact, rinse eyes throughly with water.
Reference: PL-0591 Artwork -Back.jpg
Directions
- Wet hands throughly with product, briskly rub together until dry.
- Always supervise children in use of this product.
Other Information
- Store at 68º to 77°F (20° to 25°C)
- May discolor certain fabrics
Reference: PL-0591 Artwork -Back.jpg
Inactive ingredients
Aloe Barbadensis oil, Fragrance, Propylene glycol, Tocopherol, Water(Aqua)
Reference: PL-0591 Artwork -Back.jpg
| PL-0591
flexi hand sanitizer gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Broder Bros Co., (107044246) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Yuyao Jessie Commodity Co., Ltd. | 529892305 | manufacture(71513-059) | |
Revised: 11/2022
Document Id: ee8c081d-b2df-42ec-e053-2995a90aa898
Set id: 51d7ea6d-a633-20f9-e054-00144ff8d46c
Version: 6
Effective Time: 20221128
Broder Bros Co.,
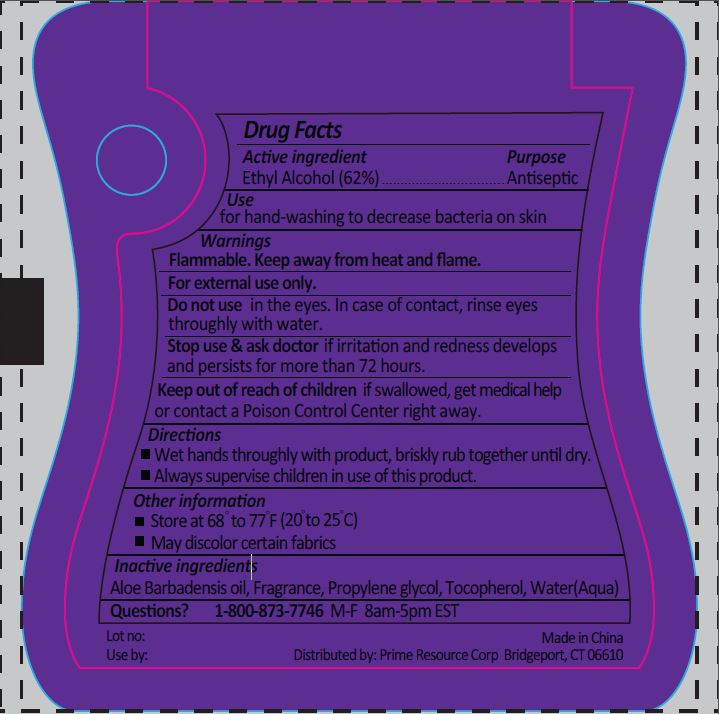
 for hand-washing to decrease bacteria on skin
for hand-washing to decrease bacteria on skin