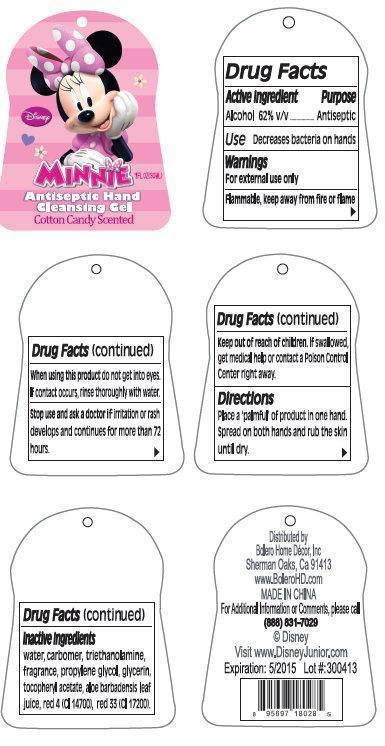
DISNEY MINNIE ANTISEPTIC HAND CLEANSING COTTON CANDY SCENTED- alcohol gel
Bolero Home Decor, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Disney Minnie Antiseptic Hand Cleansing Gel Cotton Candy Scented
Directions
Place a 'palmful' of product in one hand. Spread on both hands and rub the skin until dry.
Inactive Ingredients
water, carbomer, triethanolamine, fragrance, propylene glycol, glycerin, tocopheryl acetate, aloe barbadensis leaf juice, fragrance, red 4 (CI 14700), red 33 (CI 17200).
| DISNEY MINNIE ANTISEPTIC HAND CLEANSING COTTON CANDY SCENTED
alcohol gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Bolero Home Decor, Inc. (009777118) |
Revised: 7/2016
Document Id: 3693a16e-43e0-6a54-e054-00144ff88e88
Set id: 515b64b8-7d54-4697-a2aa-5af4511189ea
Version: 5
Effective Time: 20160701
Bolero Home Decor, Inc.