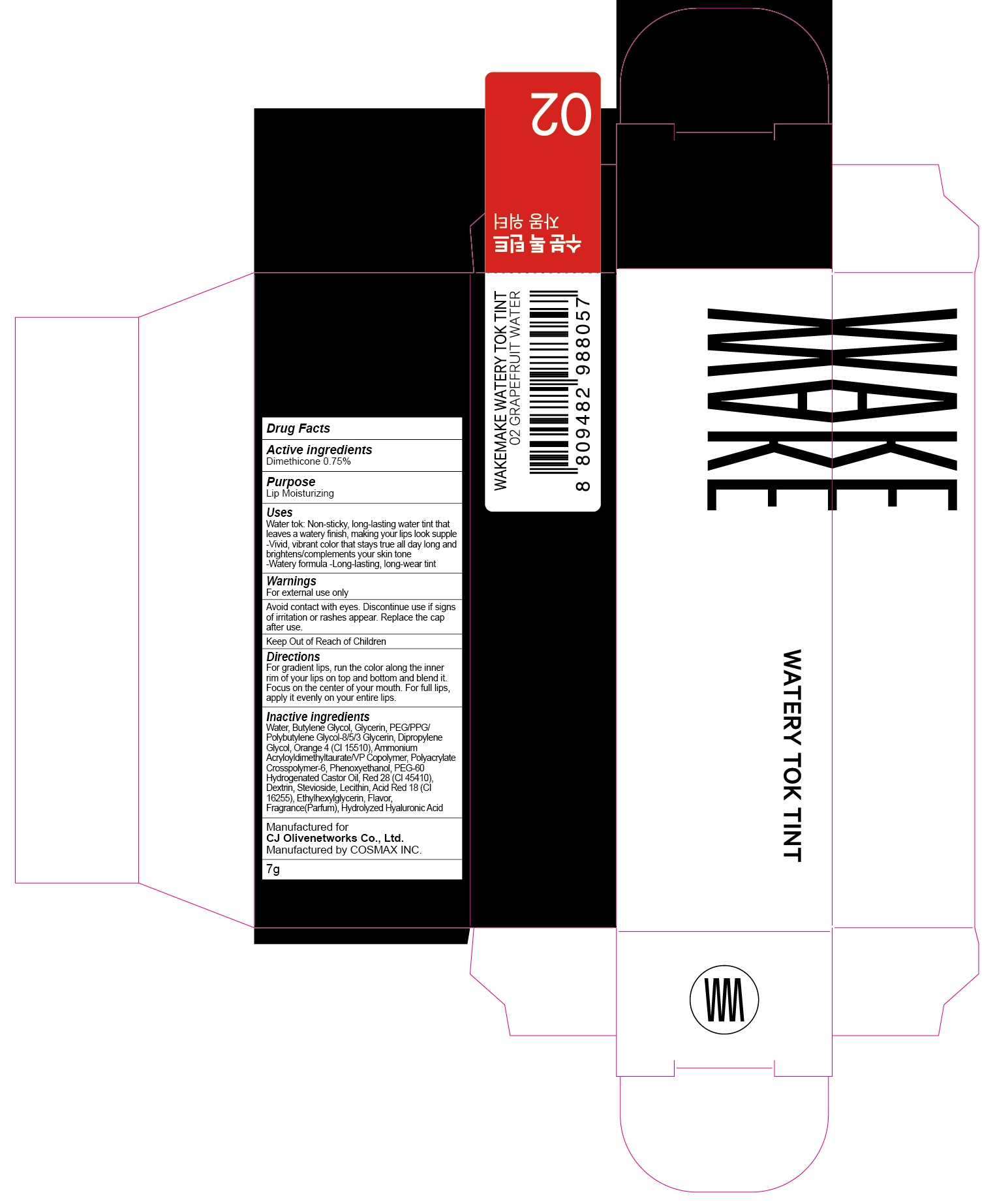
ACTIVE INGREDIENT
Active ingredients: Dimethicone 0.75%
INACTIVE INGREDIENT
Inactive ingredients: Water, Butylene Glycol, Glycerin, PEG/PPG/Polybutylene Glycol-8/5/3 Glycerin, Dipropylene Glycol, Orange 4 (CI 15510), Ammonium Acryloyldimethyltaurate/VP Copolymer, Polyacrylate Crosspolymer-6, Phenoxyethanol, PEG-60 Hydrogenated Castor Oil, Red 28 (CI 45410), Dextrin, Stevioside, Lecithin, Acid Red 18 (CI 16255), Ethylhexylglycerin, Flavor, Fragrance(Parfum), Hydrolyzed Hyaluronic Acid
PURPOSE
Purpose: Lip Moisturizing
WARNINGS
Warnings: For external use only. Avoid contact with eyes. Discontinue use if signs of irritation or rashes appear. Replace the cap after use. Keep Out of Reach of Children.
KEEP OUT OF REACH OF CHILDREN
KEEP OUT OF REACH OF CHILDREN
Uses
Uses: Water tok: Non-sticky, long-lasting water tint that leaves a watery finish, making your lips look supple -Vivid, vibrant color that stays true all day long and brightens/complements your skin tone -Watery formula -Long-lasting, long-wear tint
Directions
Directions: For gradient lips, run the color along the inner rim of your lips on top and bottom and blend it. Focus on the center of your mouth. For full lips, apply it evenly on your entire lips.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
CJ OliveNetworks Co., Ltd.
