PVP-IODINE SWABSTICK THREES- povidone-iodine swab
Dukal Corporation
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
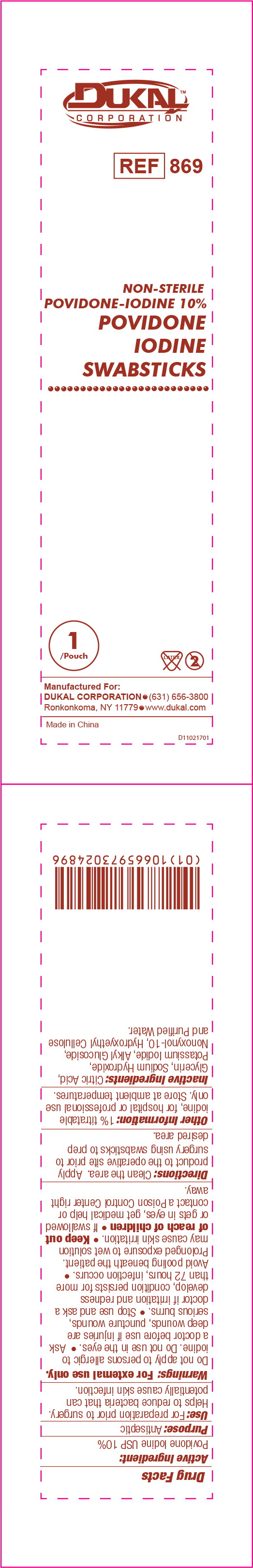
Drug Facts
Use:
For preparation prior to surgery. Helps to reduce bacteria that can potentially cause skin infection.
Warnings:
For external use only.
- Do not apply to persons allergic to iodine. Do not use in the eyes.
Directions:
Clean the area. Apply product to the operative site prior to surgery using sponge sticks to prep desired area.
Other Information:
1% titratable iodine, for hospital or professional use only.
Store at ambient temperatures.
Inactive Ingredients:
Citric Acid, Glycerin, Sodium Hydroxide, Potassium Iodide, Alkyl Glucoside, Nonoxynol-10, Hydroxyethyl Cellulose and Purified Water.
Principal Display Panel - PVP-I Prep Swabstick 869-1000 Case Label
DUKAL™
CORPORATION
REF 869-1000
NON-STERILE POVIDONE-IODINE 10%
POVIDONE-IODINE
SWABSTICKS
1000/Case
1/Pack
Manufactured For: DUKAL CORPORATION • (631) 656-3800
Ronkonkoma, NY 11779 • www.dukal.com
Made in China, Hecho en China, Fabriqué en Chine
(01) 40665973024897
Principal Display Panel - PVP-I Prep Swabstick 869-1000 Pouch Label
DUKAL™
CORPORATION
REF 869
NON-STERILE
POVIDONE-IODINE 10%
POVIDONE
IODINE
SWABSTICKS
1
/Pouch
Manufactured For:
DUKAL CORPORATION • (631) 656-3800
Ronkonkoma, NY 11779 • www.dukal.com
Made in China
D11021701
Principal Display Panel - PVP-I Prep Swabstick 867-1000 Case Label
DUKAL™
CORPORATION
REF 867-500
NON-STERILE POVIDONE-IODINE 10%
POVIDONE-IODINE
SWABSTICKS
500/Case
3/Pack
Manufactured For: DUKAL CORPORATION • (631) 656-3800
Ronkonkoma, NY 11779 • www.dukal.com
Made in China, Hecho en China, Fabriqué en Chine
(01) 50665973024658
Principal Display Panel - PVP-I Prep Swabstick 867-1000 Pouch Label
DUKAL™
CORPORATION
REF 867
NON-STERILE
POVIDONE-IODINE 10%
POVIDONE-IODINE
SWABSTICKS
3
/Pouch
Manufactured For:
DUKAL CORPORATION • (631) 656-3800
Ronkonkoma, NY 11779 • www.dukal.com
Made in China
D02231206 Rev1
Principal Display Panel - PVP-I Prep Swabstick 867 Case Label
DUKAL™
CORPORATION
REF 867
NON-STERILE POVIDONE-IODINE 10%
POVIDONE-IODINE
SWABSTICKS
10 Boxes/Case
25 Packs/Box
3/Pack
Manufactured For: DUKAL CORPORATION • (631) 656-3800
Ronkonkoma, NY 11779 • www.dukal.com
Made in China, Hecho en China, Fabriqué en Chine
(01) 40665973024651
Principal Display Panel - PVP-I Prep Swabstick 867 Carton Label
DUKAL™
CORPORATION
REF 867
NON-STERILE POVIDONE-IODINE 10%
POVIDONE-IODINE
SWABSTICKS
25
/box
3/Pouch
Manufactured For: DUKAL CORPORATION • (631) 656-3800
Ronkonkoma, NY 11779 • www.dukal.com
Made in China, Hecho en China, Fabriqué en Chine
D02231207 Rev1
| PVP-IODINE SWABSTICK THREES
povidone-iodine swab |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Dukal Corporation (791014871) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Changzhou Maokang Medical Products Co.,Ltd | 421317073 | manufacture(65517-0030) | |