SYNCOL- acetaminophen, pamabrom and pyrilamine maleate capsule
MarcasUSA LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
SYNCOL
Acetaminophen 500 mg ..........................................Pain reliever
Pamabrom 25 mg ....................................................Diuretic
Pyrilamine maleate15 mg........................................Antihistamine
Uses
for the temporary relief of these symptoms associated with menstrual periods:
- cramps
- headache
- bloating
- backache
- water-weight gain
- muscular aches
- irritability
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 8 caplets in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Do not use
with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Ask a doctor before use if you have
- liver disease
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
Ask a doctor or pharmacist before use if you are
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers
When using this product
- you may get drowsy, avoid alcoholic beverages
- alcohol sedatives and tranquilizers may increase drowsiness
- use caution when driving or operating machinery
- excitability may occur, especially in children
Directions
- adults and children 12 years and over:
- take 2 caplets with water every 6 hours as needed
- do not exceed 8 caplets in a 24 hour period or as directed by a doctor
- do not use more than directed (see warnings)
- children under 12 years:
- consult a doctor
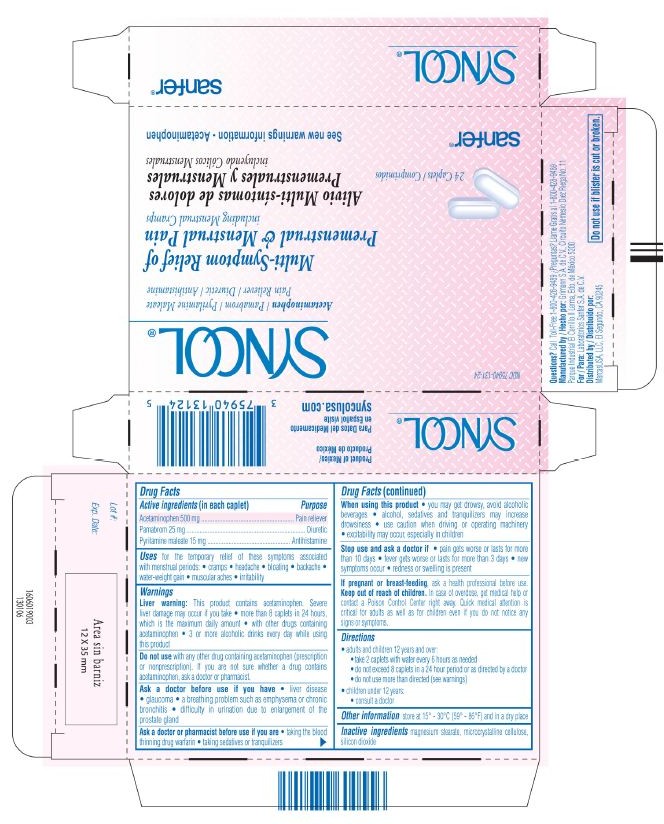
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL - 24 Capsules
NDC 75940-131-24
SYNCOL ®
Acetaminophen / Pamabrom / Pyrilamine Maleate
Pain Reliever / Diuretic / Antihistamine
Multi-Symptom Relief of
Premenstrual & Menstrual Pain
including Menstrual Cramps
Alivio Multi-síntomas de dolores
Premenstruales y Menstruales
incluyendo Cólicos Menstruales
24 Caplets / Comprimidos
See new warnings information - Acetaminophen
sanfer
Carton
| SYNCOL
acetaminophen, pamabrom, pyrilamine maleate capsule |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - MarcasUSA LLC (016139820) |