CEFOTAXIME- cefotaxime injection, powder, for solution
Lupin Pharmaceuticals, Inc.
----------
CEFOTAXIME FOR INJECTION USP
500 mg, 1 g and 2 g
Rx only
DESCRIPTION
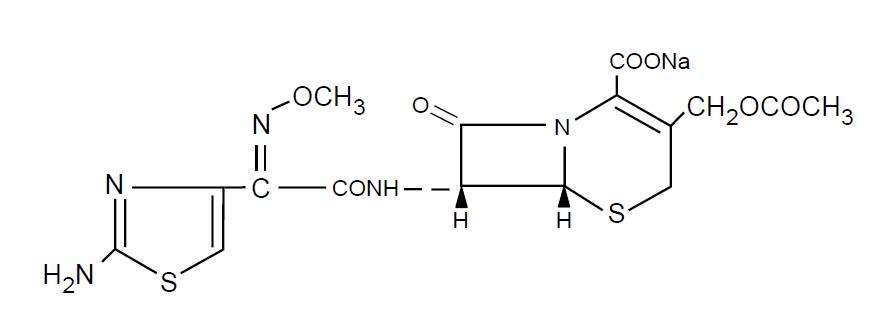
Cefotaxime for Injection USP is a semisynthetic, broad spectrum cephalosporin antibiotic for parenteral administration. It is the sodium salt of 7-[2-(2-amino-4-thiazolyl) glyoxylamido]-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo [4.2.0] oct-2-ene-2-carboxylate 72 (Z)-(o-methyloxime), acetate (ester). Cefotaxime for injection USP contains approximately 50.5 mg (2.2 mEq) of sodium per gram of cefotaxime activity. Solutions of cefotaxime for injection USP range from very pale yellow to light amber depending on the concentration and the diluent used. The pH of the injectable solutions usually ranges from 5 to 7.5. The CAS Registry Number is 64485-93-4.
Cefotaxime for injection USP is supplied as a dry powder in conventional vials. Each vial contains cefotaxime sodium equivalent to 500 mg, 1 g or 2 g of cefotaxime.
CLINICAL PHARMACOLOGY
Following IM administration of a single 500 mg or 1 g dose of cefotaxime for injection to normal volunteers, mean peak serum concentrations of 11.7 and 20.5 mcg/mL respectively were attained within 30 minutes and declined with an elimination half-life of approximately 1 hour. There was a dose-dependent increase in serum levels after the IV administration of 500 mg, 1 g, and 2 g of cefotaxime for injection (38.9, 101.7, and 214.4 mcg/mL respectively) without alteration in the elimination half-life. There is no evidence of accumulation following repetitive IV infusion of 1 g doses every 6 hours for 14 days as there are no alterations of serum or renal clearance. About 60% of the administered dose was recovered from urine during the first 6 hours following the start of the infusion.
Approximately 20 to 36% of an intravenously administered dose of 14C-cefotaxime is excreted by the kidney as unchanged cefotaxime and 15 to 25% as the desacetyl derivative, the major metabolite. The desacetyl metabolite has been shown to contribute to the bactericidal activity. Two other urinary metabolites (M2 and M3) account for about 20 to 25%. They lack bactericidal activity.
A single 50 mg/kg dose of cefotaxime for injection was administered as an intravenous infusion over a 10- to 15minute period to 29 newborn infants grouped according to birth weight and age. The mean half-life of cefotaxime in infants with lower birth weights (≤1500 grams), regardless of age, was longer (4.6 hours) than the mean half-life (3.4 hours) in infants whose birth weight was greater than 1500 grams. Mean serum clearance was also smaller in the lower birth weight infants. Although the differences in mean half-life values are statistically significant for weight, they are not clinically important. Therefore, dosage should be based solely on age. (See DOSAGE AND ADMINISTRATION section.)
Drug Interactions
A single intravenous dose and oral dose of probenecid (500 mg each) followed by two oral doses of probenecid 500 mg at approximately hourly intervals administered to three healthy male subjects receiving a continuous infusion of cefotaxime increased the steady-state plasma concentration of cefotaxime by approximately 80%. In another study, administration of oral probenecid 500 mg every 6 hours to six healthy male subjects with cefotaxime 1 gram infused over 5 minutes decreased the total clearance of cefotaxime by approximately 50%.
Additionally, no disulfiram-like reactions were reported in a study conducted in 22 healthy volunteers administered cefotaxime for injection and ethanol.
Microbiology
Cefotaxime sodium is a bactericidal agent that acts by inhibition of bacterial cell wall synthesis. Cefotaxime has activity in the presence of some beta-lactamases, both penicillinases and cephalosporinases, of Gram-negative and Gram-positive bacteria.
Mechanism of Resistance
Resistance to cefotaxime is primarily through hydrolysis by beta-lactamase, alteration of penicillin-binding proteins (PBPs), and decreased permeability.
Susceptibility to cefotaxime will vary geographically and may change over time; local susceptibility data should be consulted, if available. Cefotaxime has been shown to be active against most isolates of the following bacteria both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section:
Gram-positive bacteria:
Enterococcus spp.1
Staphylococcus aureus (methicillin-susceptible isolates only)
Staphylococcus epidermidis
Streptococcus pneumoniae
Streptococcus pyogenes (Group A beta-hemolytic streptococci)
Streptococcus spp. (Viridans group streptococci)
Gram-negative bacteria:
Acinetobacter spp.
Citrobacter spp.2
Enterobacter spp.2
Escherichia coli.2
Haemophilus influenzae
Haemophilus parainfluenzae
Klebsiella spp. (including Klebsiella pneumoniae)2
Morganella morganii2
Neisseria gonorrhoeae (including beta-lactamase-positive and negative strains)
Neisseria meningitidis
Proteus mirabilis2
Proteus vulgaris2
Providencia rettgeri2
Providencia stuartii2
Serratia marcescens2
1Enterococcus species may be intrinsically resistant to cefotaxime.
2Most extended spectrum beta-lactamase (ESBL)-producing and carbapenemase-producing isolates are resistant to cefotaxime.
Anaerobic bacteria:
Bacteroides spp., including some isolates of Bacteroides fragilis
Clostridium spp. (most isolates of Clostridium difficile are resistant)
Fusobacterium spp. (including Fusobacterium nucleatum)
Peptococcus spp. Peptostreptococcus spp.
The following in vitro data are available, but their clinical significance is unknown. At least 90 percent of the following microorganisms exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to 1 mcg/mL. However, the efficacy of cefotaxime in treating clinical infections due to these microorganisms has not been established in adequate and well-controlled clinical trials.
Gram-negative bacteria:
Providencia spp.
Salmonella spp. (including Salmonella typhi)
Shigella spp.
Susceptibility Test Methods
When available, the clinical microbiology laboratory should provide the results of in vitro susceptibility test results for antimicrobial drug products used in resident hospitals to the physician as periodic reports that describe the susceptibility profile of nosocomial and community-acquired pathogens. These reports should aid the physician in selecting an antibacterial drug product for treatment.
Dilution techniques:
Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized test method (broth or agar)1,2 . The MIC values should be interpreted according to the criteria provided in Table 1.
Diffusion techniques:
Quantitative methods that require measurement of zone diameters also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. The zone size provides an estimate of the susceptibility of bacteria to antimicrobial compounds. The zone size should be determined using a standardized test method2,3. This procedure uses paper disks impregnated with 30 mcg cefotaxime to test the susceptibility of microorganisms to cefotaxime. The disk diffusion interpretive criteria are provided in Table 1.
Anaerobic techniques:
For anaerobic bacteria, the susceptibility to cefotaxime as MICs can be determined by a standardized agar test method3,4. The MIC values obtaine d should be interpreted according to the criteria provided in Table 1.
|
Susceptible breakpoints are based on a dose of 1 gram q 8h in patients with normal renal function |
||||||
|
Susceptibility of staphylococci to cefotaxime may be deduced from testing only penicillin and either cefoxitin or oxacillin. |
||||||
|
||||||
|
| Minimum Inhibitory Concentrations (mcg/mL)
| Disk Diffusion Zone Diameters (mm)
|
||||
| Pathogen
| (S) Susceptible
| (I) Intermediate
| (R) Resistant
| (S) Susceptible
| (I) Intermediate
| (R) Resistant
|
| Acinetobacter spp.
| ≤1 | 2 | ≥4 |
|
|
|
| Enterobacteriaceae
| ≤1 | 2 | ≥4 | ≥26 | 23 to 25 | ≤22 |
| Haemophilus spp. * †
| ≤1 | - | - |
| - | - |
| Neisseria gonorrhoeae*
| ≤0.5 | - | - | ≥31 | - | - |
| Neisseria meningitidis*
| ≤0.12 | - | - | ≥34 | - | - |
| Streptococcus pneumoniae‡ meningitis isolates | ≤0.5 | 1 | ≥2 | - | - | - |
| Streptococcus pneumoniae‡ non-meningitis isolates | ≤1 | 2 | ≥ | - | - | - |
| Streptococcus spp. beta-hemolytic group* | ≤0.5 | - | - | ≥24 | - | - |
| Viridans group streptococci | ≤1 | 2 | ≥4 | ≥28 | 26 to 27 | ≤25 |
| Other Non-Enterobacteriaceae§
| ≤1 | 2 | ≥4 |
|
|
|
| Anaerobic bacteria (agar method) | ≤1 | 2 | ≥4 | - | - | - |
A report of Susceptible indicates that the antimicrobial drug is likely to inhibit growth of the pathogen if the antimicrobial drug reaches the concentration at the site of infection. A report of Intermediate indicates that the result should be considered equivocal, and if the microorganism is not fully susceptible to alternative, clinically feasible drugs, the test should be repeated. This category implies possible clinical applicability in body sites where the drug is physiologically concentrated or in situations where a high dosage of drug can be used. This category also provides a buffer zone that prevents small uncontrolled technical factors from causing major discrepancies in interpretation. A report of Resistant indicates that the antimicrobial drug is not likely to inhibit growth of the pathogen if the antimicrobial drug reaches the concentrations usually achievable at the site of infection; other therapy should be selected.
Quality Control:
Standardized susceptibility test procedures require the use of laboratory controls to monitor and ensure the accuracy and precision of supplies and reagents used in the assay, and the techniques of the individual performing the test1,2,3,4. Standard cefotaxime powder should provide the following range of MIC values noted in Table 2. For the diffusion technique using the 30 mcg disk, the criteria in Table 2 should be achieved.
|
||
| QC Strain
| Minimum Inhibitory Concentrations (mcg/mL)
| Disk Diffusion Zone Diameters (mm)
|
| Escherichia coli ATCC 25922
| 0.03 to 0.12 | 29 to 35 |
| Staphylococcus aureus ATCC 29213
| 1 to 4 | - |
| Staphylococcus aureus ATCC 25923
| - | 25 to 31 |
| Pseudomonas aeruginosa ATCC 27853
| 8 to 32 | 18 to 22 |
| Haemophilus influenzae ATCC 49247
| 0.12 to 0.5 | 31 to 39 |
| Streptococcus pneumoniae ATCC 49619
| 0.03 to 0.12 | 31 to 39 |
| Neisseria gonorrhoeae ATCC 49226
| 0.015 to 0.06 | 38 to 48 |
| Bacteroides fragilis* ATCC 25285
| 8 to 32 | - |
| Bacteroides thetaiotaomicron* ATCC 29741
| 16 to 64 | - |
| Eubacterium lantem* ATCC 43055
| 64 to 256 | - |
INDICATIONS AND USAGE
Treatment
Cefotaxime for Injection USP is indicated for the treatment of patients with serious infections caused by susceptible strains of the designated microorganisms in the diseases listed below.
- Lowerrespiratorytractinfections, including pneumonia, caused byStreptococcuspneumoniae(formerlyDiplococcuspneumoniae),Streptococcuspyogenes* (Group A streptococci) and other streptococci (excluding enterococci, e.g.,Enterococcusfaecalis),Staphylococcusaureus(penicillinase and non-penicillinase producing),Escherichiacoli,Klebsiellaspecies,Haemophilusinfluenzae(including ampicillin resistant strains),Haemophilusparainfluenzae,Proteusmirabilis,Serratiamarcescens*,Enterobacterspecies, indole positiveProteusandPseudomonasspecies (includingP.aeruginosa).
- Genitourinaryinfections. Urinary tract infections caused byEnterococcusspecies,Staphylococcusepidermidis,Staphylococcusaureus*, (penicillinase and non-penicillinase producing),Citrobacterspecies,Enterobacterspecies,Escherichiacoli,Klebsiellaspecies,Proteusmirabilis,Proteusvulgaris*,Providenciastuartii,Morganellamorganii*,Providenciarettgeri*,SerratiamarcescensandPseudomonasspecies (includingP.aeruginosa). Also, uncomplicated gonorrhea (cervical/urethral and rectal) caused byNeisseriagonorrhoeae, including penicillinase producing strains.
- Gynecologicinfections, including pelvic inflammatory disease, endometritis and pelvic cellulitis caused byStaphylococcusepidermidis,Streptococcusspecies,Enterococcusspecies,Enterobacterspecies*,Klebsiellaspecies*,Escherichiacoli,Proteusmirabilis,Bacteroidesspecies (includingBacteroidesfragilis*),Clostridiumspecies, and anaerobic cocci (includingPeptostreptococcusspecies andPeptococcusspecies) andFusobacteriumspecies (includingF.nucleatum*). Cefotaxime for Injection USP, like other cephalosporins, has no activity againstChlamydiatrachomatis. Therefore, when cephalosporins are used in the treatment of patients with pelvic inflammatory disease andC.trachomatisis one of the suspected pathogens, appropriate anti-chlamydial coverage should be added.
- Bacteremia/Septicemiacaused byEscherichiacoli,Klebsiellaspecies, andSerratiamarcescens,StaphylococcusaureusandStreptococcusspecies (includingS.pneumonia).
- Skinandskinstructureinfectionscaused byStaphylococcusaureus(penicillinase and nonpenicillinase producing),Staphylococcusepidermidis,Streptococcuspyogenes(Group A streptococci) and other streptococci,Enterococcusspecies,Acinetobacterspecies*,Escherichiacoli,Citrobacterspecies (includingC.freundii*),Enterobacterspecies,Klebsiellaspecies,Proteusmirabilis,Proteusvulgaris*,Morganellamorganii,Providenciarettgeri*,Pseudomonasspecies,Serratiamarcescens,Bacteroidesspecies, and anaerobic cocci (includingPeptostreptococcus*species andPeptococcusspecies).
- Intra-abdominalinfectionsincluding peritonitis caused byStreptococcusspecies*,Escherichiacoli,Klebsiellaspecies,Bacteroidesspecies, and anaerobic cocci (includingPeptostreptococcus*species andPeptococcus*species)Proteusmirabilis*, andClostridiumspecies*.
- Boneand/orjointinfectionscaused byStaphylococcusaureus(penicillinase and non-penicillinase producing strains),Streptococcusspecies (includingS.pyogenes*),Pseudomonasspecies (includingP.aeruginosa*), andProteusmirabilis*.
- Centralnervoussysteminfections, e.g., meningitis and ventriculitis, caused byNeisseriameningitidis,Haemophilusinfluenzae,Streptococcuspneumoniae,Klebsiellapneumoniae*andEscherichiacoli*.
(*) Efficacy for this organism, in this organ system, has been studied in fewer than 10 infections.
Although many strains of enterococci (e.g., S. faecalis) and Pseudomonas species are resistant to cefotaxime sodium in vitro, cefotaxime for injection USP has been used successfully in treating patients with infections caused by susceptible organisms.
Specimens for bacteriologic culture should be obtained prior to therapy in order to isolate and identify causative organisms and to determine their susceptibilities to cefotaxime for injection USP. Therapy may be instituted before results of susceptibility studies are known; however, once these results become available, the antibiotic treatment should be adjusted accordingly.
In certain cases of confirmed or suspected gram-positive or gram-negative sepsis or in patients with other serious infections in which the causative organism has not been identified, cefotaxime for injection USP may be used concomitantly with an aminoglycoside. The dosage recommended in the labeling of both antibiotics may be given and depends on the severity of the infection and the patient's condition. Renal function should be carefully monitored, especially if higher dosages of the aminoglycosides are to be administered or if therapy is prolonged, because of the potential nephrotoxicity and ototoxicity of aminoglycoside antibiotics. It is possible that nephrotoxicity may be potentiated if cefotaxime for injection USP is used concomitantly with an aminoglycoside.
Prevention
The administration of cefotaxime for injection USP preoperatively reduces the incidence of certain infections in patients undergoing surgical procedures (e.g., abdominal or vaginal hysterectomy, gastrointestinal and genitourinary tract surgery) that may be classified as contaminated or potentially contaminated.
In patients undergoing cesarean section, intraoperative (after clamping the umbilical cord) and postoperative use of cefotaxime for injection USP may also reduce the incidence of certain postoperative infections. See DOSAGE AND ADMINISTRATION section.
Effective use for elective surgery depends on the time of administration. To achieve effective tissue levels, cefotaxime for injection USP should be given ½ or 1½ hours before surgery. See DOSAGE AND ADMINISTRATION section.
For patients undergoing gastrointestinal surgery, preoperative bowel preparation by mechanical cleansing as well as with a non-absorbable antibiotic (e.g., neomycin) is recommended.
If there are signs of infection, specimens for culture should be obtained for identification of the causative organism so that appropriate therapy may be instituted.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefotaxime for injection USP and other antibacterial drugs, cefotaxime for injection USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
WARNINGS
BEFORE THERAPY WITH CEFOTAXIME FOR INJECTION IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEFOTAXIME SODIUM, CEPHALOSPORINS, PENICILLINS, OR OTHER DRUGS. THIS PRODUCT SHOULD BE GIVEN WITH CAUTION TO PATIENTS WITH TYPE I HYPERSENSITIVITY REACTIONS TO PENICILLIN. ANTIBIOTICS SHOULD BE ADMINISTERED WITH CAUTION TO ANY PATIENT WHO HAS DEMONSTRATED SOME FORM OF ALLERGY, PARTICULARLY TO DRUGS. IF AN ALLERGIC REACTION TO CEFOTAXIME FOR INJECTION OCCURS, DISCONTINUE TREATMENT WITH THE DRUG. SERIOUS HYPERSENSITIVITY REACTIONS MAY REQUIRE EPINEPHRINE AND OTHER EMERGENCY MEASURES.
During post-marketing surveillance, a potentially life-threatening arrhythmia was reported in each of six patients who received a rapid (less than 60 seconds) bolus injection of cefotaxime through a central venous catheter. Therefore, cefotaxime should only be administered as instructed in the DOSAGE AND ADMINISTRATION section.
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including cefotaxime for injection, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
PRECAUTIONS
General
Prescribing cefotaxime for injection in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Cefotaxime for injection should be prescribed with caution in individuals with a history of gastrointestinal disease, particularly colitis.
Because high and prolonged serum antibiotic concentrations can occur from usual doses in patients with transient or persistent reduction of urinary output because of renal insufficiency, the total daily dosage should be reduced when cefotaxime for injection is administered to such patients. Continued dosage should be determined by degree of renal impairment, severity of infection, and susceptibility of the causative organism.
Although there is no clinical evidence supporting the necessity of changing the dosage of cefotaxime sodium in patients with even profound renal dysfunction, it is suggested that, until further data are obtained, the dose of cefotaxime sodium be halved in patients with estimated creatinine clearances of less than 20 mL/min/1.73 m2 .
When only serum creatinine is available, the following formula5 (based on sex, weight, and age of the patient) may be used to convert this value into creatinine clearance. The serum creatinine should represent a steady state of renal function.
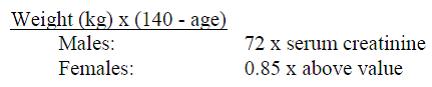
As with other antibiotics, prolonged use of cefotaxime for injection may result in overgrowth of nonsusceptible organisms. Repeated evaluation of the patient's condition is essential. If superinfection occurs during therapy, appropriate measures should be taken.
Leukopenia, neutropenia, granulocytopenia and, more rarely, bone marrow failure, pancytopenia, or agranulocytosis may develop during treatment with cefotaxime for injection USP. For courses of treatment lasting longer than 10 days, blood counts should therefore be monitored and treatment discontinuation should be considered in case of abnormal results.
Cefotaxime for injection, like other parenteral anti-infective drugs, may be locally irritating to tissues. In most cases, perivascular extravasation of cefotaxime for injection responds to changing of the infusion site. In rare instances, extensive perivascular extravasation of cefotaxime for injection may result in tissue damage and require surgical treatment. To minimize the potential for tissue inflammation, infusion sites should be monitored regularly and changed when appropriate.
Information for patients
Patients should be counseled that antibacterial drugs including cefotaxime for injection should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When cefotaxime for injection is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by cefotaxime for injection or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Drug Interactions
As with other cephalosporins, cefotaxime for injection may potentiate the nephrotoxic effects of nephrotoxic drugs such as aminoglycosides, NSAIDs and furosemide.
Probenecid interferes with the renal tubular transfer of cefotaxime, decreasing the total clearance of cefotaxime by approximately 50% and increasing the plasma concentrations of cefotaxime. Administration of cefotaxime in excess of 6 grams/day should be avoided in patients receiving probenecid (see CLINICAL PHARMACOLOGY, Drug Interactions).
Drug/Laboratory Test Interactions
Cephalosporins, including cefotaxime sodium, are known to occasionally induce a positive direct Coombs' test.
A false-positive reaction for glucose in the urine may occur with copper reduction tests (Benedict's or Fehling's solution or with CLINITEST tablets), but not with enzyme-based tests for glycosuria. (e.g., CLINISTIX or TesTape). There are no reports in published literature that link elevations of plasma glucose levels to the use of cefotaxime.
Carcinogenesis, Mutagenesis
Lifetime studies in animals to evaluate carcinogenic potential have not been conducted. Cefotaxime for injection was not mutagenic in the mouse micronucleus test or in the Ames test. Cefotaxime for injection did not impair fertility to rats when administered subcutaneously at doses up to 250 mg/kg/day (0.2 times the maximum recommended human dose based on mg/m2) or in mice when administered intravenously at doses up to 2000 mg/kg/day (0.7 times the recommended human dose based on mg/m2).
Pregnancy
Pregnancy Category B:
Reproduction studies have been performed in pregnant mice given cefotaxime for injection intravenously at doses up to 1200 mg/kg/day (0.4 times the recommended human dose based on mg/m2) or in pregnant rats when administered intravenously at doses up to 1200 mg/kg/day (0.8 times the recommended human dose based on mg/m2). No evidence of embryotoxicity or teratogenicity was seen in these studies. Although cefotaxime has been reported to cross the placental barrier and appear in cord blood, the effect on the human fetus is not known. There are no well-controlled studies in pregnant women. Because animal reproductive studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nonteratogenic Effects
Use of the drug in women of child-bearing potential requires that the anticipated benefit be weighed against the possible risks.
In perinatal and postnatal studies with rats, the pups in the group given 1200 mg/kg/day of cefotaxime were significantly lighter in weight at birth and remained smaller than pups in the control group during the 21 days of nursing.
Nursing Mothers
Cefotaxime is excreted in human milk in low concentrations. Caution should be exercised when cefotaxime is administered to a nursing woman.
Geriatric Use
Of the 1409 subjects in clinical studies of cefotaxime, 632 (45%) were 65 and over, while 258 (18%) were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function (see PRECAUTIONS, General).
ADVERSE REACTIONS
Clinical Trials Experience
Cefotaxime for injection is generally well tolerated. The most common adverse reactions have been local reactions following IM or IV injection. Other adverse reactions have been encountered infrequently.
The most frequent adverse reactions (greater than 1%) are:
Local (4.3%) - Injection site inflammation with IV administration. Pain, induration, and tenderness after IM injection.
Hypersensitivity (2.4%) - Rash, pruritus, fever, eosinophilia.
Gastrointestinal (1.4%) - Colitis, diarrhea, nausea, and vomiting.
Symptoms of pseudomembranous colitis can appear during or after antibiotic treatment.
Nausea and vomiting have been reported rarely.
Less frequent adverse reactions (less than 1%) are:
Hematologic System - Neutropenia, leukopenia, have been reported. Some individuals have developed positive direct Coombs Tests during treatment with cefotaxime for injection and other cephalosporin antibiotics.
Genitourinary System - Moniliasis, vaginitis.
Central Nervous System - Headache. Liver - Transient elevations in AST, ALT, serum LDH, and serum alkaline phosphatase levels have been reported.
Kidney - As with some other cephalosporins, transient elevations of BUN have been occasionally observed with cefotaxime for injection.
Post-Marketing Experience
The following adverse reactions have been identified during post-approval use of cefotaxime for injection. Because these reactions were reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiovascular System - Potentially life-threatening arrhythmias following rapid (less than 60 seconds) bolus administration via central venous catheter have been observed.
Central Nervous System - Administration of high doses of beta-lactam antibiotics, including cefotaxime, particularly in patients with renal insufficiency may result in encephalopathy (e.g. impairment of consciousness, abnormal movements and convulsions). Dizziness has also been reported.
Cutaneous - As with other cephalosporins, isolated cases of toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme have been reported. Acute generalized exanthematous pustulosis (AGEP) has also been reported.
General disorders and administration site conditions - Inflammatory reactions at the injection site, including phlebitis/thrombophlebitis.
Hematologic System - Hemolytic anemia, agranulocytosis, thrombocytopenia, pancytopenia, bone marrow failure.
Hypersensitivity - Anaphylaxis (e.g., angioedema, bronchospasm, malaise possibly culminating in shock), urticaria.
Kidney - Interstitial nephritis, transient elevations of creatinine, acute renal failure.
Liver - Hepatitis, jaundice, cholestasis, elevations of gamma GT and bilirubin.
Cephalosporin Class Labeling
In addition to the adverse reactions listed above which have been observed in patients treated with cefotaxime sodium, the following adverse reactions and altered laboratory tests have been reported for cephalosporin class antibiotics: allergic reactions, hepatic dysfunction including cholestasis, aplastic anemia, hemorrhage, and false-positive test for urinary glucose.
Several cephalosporins have been implicated in triggering seizures, particularly in patients with renal impairment when the dosage was not reduced. See DOSAGE AND ADMINISTRATION and OVERDOSAGE. If seizures associated with drug therapy occur, the drug should be discontinued. Anticonvulsant therapy can be given if clinically indicated.
To report SUSPECTED ADVERSE REACTIONS, contact Lupin Pharmaceuticals, Inc. at 1-800-399-2561 or FDA at 1-800-FDA-1088 or www.fda.gov.
OVERDOSAGE
The acute toxicity of cefotaxime was evaluated in neonatal and adult mice and rats. Significant mortality was seen at parenteral doses in excess of 6000 mg/kg/day in all groups. Common toxic signs in animals that died were a decrease in spontaneous activity, tonic and clonic convulsions, dyspnea, hypothermia, and cyanosis. Cefotaxime sodium overdosage has occurred in patients. Most cases have shown no overt toxicity. The most frequent reactions were elevations of BUN and creatinine. There is a risk of reversible encephalopathy in cases of administration of high doses of beta-lactam antibiotics including cefotaxime. No specific antidote exists. Patients who receive an acute overdosage should be carefully observed and given supportive treatment.
DOSAGE AND ADMINISTRATION
Adults
Dosage and route of administration should be determined by susceptibility of the causative organisms, severity of the infection, and the condition of the patient (see table for dosage guideline). Cefotaxime for injection may be administered IM or IV after reconstitution. The maximum daily dosage should not exceed 12 grams.
| Type of Infection
| Daily Dose
(grams) | Frequency and Route
|
| Gonococcal urethritis/ cervicitis in males and females
| 0.5
| 0.5 gram IM (single dose)
|
| Rectal gonorrhea in females
| 0.5
| 0.5 gram IM (single dose)
|
| Rectal gonorrhea in males
| 1 | 1 gram IM (single dose)
|
| Uncomplicated infections
| 2 | 1 gram every 12 hours IM or IV
|
| Moderate to severe infections
| 3 to 6 | 1 to 2 grams every 8 hours IM or IV
|
| Infections commonly needing antibiotics in higher dosage (e.g., septicemia)
| 6 to 8 | 2 grams every 6 to 8 hours IV
|
| Life-threatening infections
| Up to 12 | 2 grams every 4 hours IV
|
If C. trachomatis is a suspected pathogen, appropriate anti-chlamydial coverage should be added, because cefotaxime sodium has no activity against this organism.
To prevent postoperative infection in contaminated or potentially contaminated surgery, the recommended dose is a single 1 gram IM or IV administered 30 to 90 minutes prior to start of surgery.
Cesarean Section Patients
The first dose of 1 gram is administered intravenously as soon as the umbilical cord is clamped. The second and third doses should be given as 1 gram intravenously or intramuscularly at 6 and 12 hours after the first dose.
Neonates, Infants, and Children
The following dosage schedule is recommended:
Neonates (birth to 1 month)
0 to 1 week of age 50 mg/kg per dose every 12 hours IV
1 to 4 weeks of age 50 mg/kg per dose every 8 hours IV
It is not necessary to differentiate between premature and normal-gestational age infants.
Infants and Children (1 month to 12 years)
For body weights less than 50 kg, the recommended daily dose is 50 to 180 mg/kg IM or IV body weight divided into four to six equal doses. The higher dosages should be used for more severe or serious infections, including meningitis. For body weights 50 kg or more, the usual adult dosage should be used; the maximum daily dosage should not exceed 12 grams.
Geriatric Use
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. (See PRECAUTIONS, General and PRECAUTIONS, Geriatric Use.)
Impaired Renal Function
see PRECAUTIONS, General.
NOTE: As with antibiotic therapy in general, administration of cefotaxime for injection should be continued for a minimum of 48 to 72 hours after the patient defervesces or after evidence of bacterial eradication has been obtained; a minimum of 10 days of treatment is recommended for infections caused by Group A beta-hemolytic streptococci in order to guard against the risk of rheumatic fever or glomerulonephritis; frequent bacteriologic and clinical appraisal is necessary during therapy of chronic urinary tract infection and may be required for several months after therapy has been completed; persistent infections may require treatment of several weeks and doses smaller than those indicated above should not be used.
Preparation of Cefotaxime for Injection
Cefotaxime for injection for IM or IV administration should be reconstituted as follows:
| Strength
| Diluent
(mL) | Withdrawable
Volume (mL) | Approximate
Concentration (mg/mL) |
| 500 mg vial* (IM) | 2 | 2.2 | 230 |
| 1g vial* (IM) | 3 | 3.4 | 300 |
| 2g vial* (IM) | 5 | 6.0 | 330 |
| 500 mg vial* (IV) | 10 | 10.2 | 50 |
| 1g vial* (IV) | 10 | 10.4 | 95 |
| 2g vial* (IV) | 10 | 11.0 | 180 |
Shake to dissolve; inspect for particulate matter and discoloration prior to use. Solutions of cefotaxime for injection range from very pale yellow to light amber, depending on concentration, diluent used, and length and condition of storage.
For intramuscular use
Reconstitute VIALS with Sterile Water for Injection or Bacteriostatic Water for Injection as described above.
For intravenous use
Reconstitute VIALS with at least 10 mL of Sterile Water for Injection. For other diluents, see COMPATIBILITY AND STABILITY section.
NOTE: Solutions of cefotaxime for injection must not be admixed with aminoglycoside solutions. If cefotaxime for injection and aminoglycosides are to be administered to the same patient, they must be administered separately and not as mixed injection.
A SOLUTION OF 1 G CEFOTAXIME FOR INJECTION IN 14 ML OF STERILE WATER FOR INJECTION IS ISOTONIC.
IM Administration
As with all IM preparations, cefotaxime for injection should be injected well within the body of a relatively large muscle such as the upper outer quadrant of the buttock (i.e., gluteus maximus); aspiration is necessary to avoid inadvertent injection into a blood vessel. Individual IM doses of 2 grams may be given if the dose is divided and is administered in different intramuscular sites.
IV Administration
The IV route is preferable for patients with bacteremia, bacterial septicemia, peritonitis, meningitis, or other severe or life-threatening infections, or for patients who may be poor risks because of lowered resistance resulting from such debilitating conditions as malnutrition, trauma, surgery, diabetes, heart failure, or malignancy, particularly if shock is present or impending.
For intermittent IV administration, a solution containing 1 gram or 2 grams in 10 mL of Sterile Water for Injection can be injected over a period of three to five minutes. Cefotaxime should not be administered over a period of less than three minutes. (See WARNINGS). With an infusion system, it may also be given over a longer period of time through the tubing system by which the patient may be receiving other IV solutions. However, during infusion of the solution containing cefotaxime, it is advisable to discontinue temporarily the administration of other solutions at the same site.
For the administration of higher doses by continuous IV infusion, a solution of cefotaxime may be added to IV bottles containing the solutions discussed below.
Compatibility and Stability
Solutions of cefotaxime for injection reconstituted as described above (Preparation of cefotaxime for injection) remain chemically stable (potency remains above 90%) as follows when stored in original containers and disposable plastic syringes:
| Strength
| Reconstituted
Concentration mg/mL | Stability at or
below 22°C | Stability under
Refrigeration (at or below 5°C) Original Containers | Plastic
Syringes |
| 500 mg vial IM | 230 | 12 hours | 7 days | 5 days |
| 1g vial IM | 300 | 12 hours | 7 days | 5 days |
| 2g vial IM | 330 | 12 hours | 7 days | 5 days |
| 500 mg vial IV | 50 | 24 hours | 7 days | 5 days |
| 1g vial IV | 95 | 24 hours | 7 days | 5 days |
| 2g vial IV | 180 | 12 hours | 7 days | 5 days |
Reconstituted solutions stored in original containers and plastic syringes remain stable for 13 weeks frozen.
Reconstituted solutions may be further diluted up to 1000 mL with the following solutions and maintain satisfactory potency for 24 hours at or below 22°C, and at least 5 days under refrigeration (at or below 5°C): 0.9% Sodium Chloride Injection; 5 or 10% Dextrose Injection; 5% Dextrose and 0.9% Sodium Chloride Injection, 5% Dextrose and 0.45% Sodium Chloride Injection; 5% Dextrose and 0.2% Sodium Chloride Injection; Lactated Ringer's Solution; Sodium Lactate Injection (M/6); 10% Invert Sugar Injection, 8.5% Travasol® (Amino Acid) Injection without Electrolytes.
NOTE: Cefotaxime for injection solutions exhibit maximum stability in the pH 5 to 7 range. Solutions of cefotaxime for injection should not be prepared with diluents having a pH above 7.5, such as Sodium Bicarbonate Injection.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
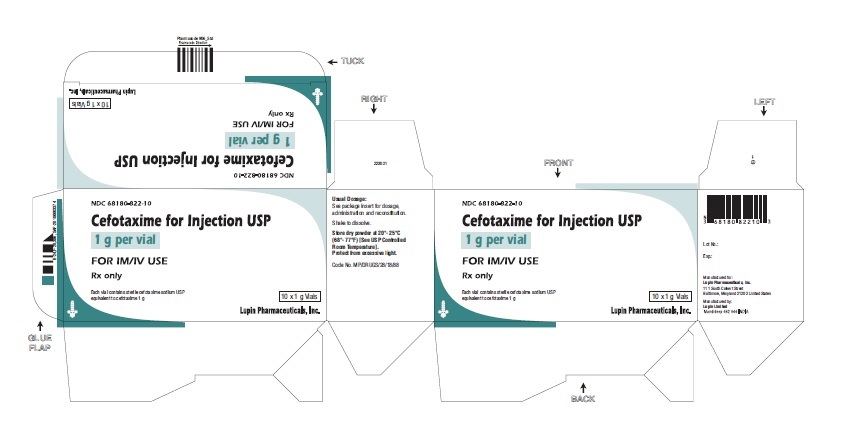
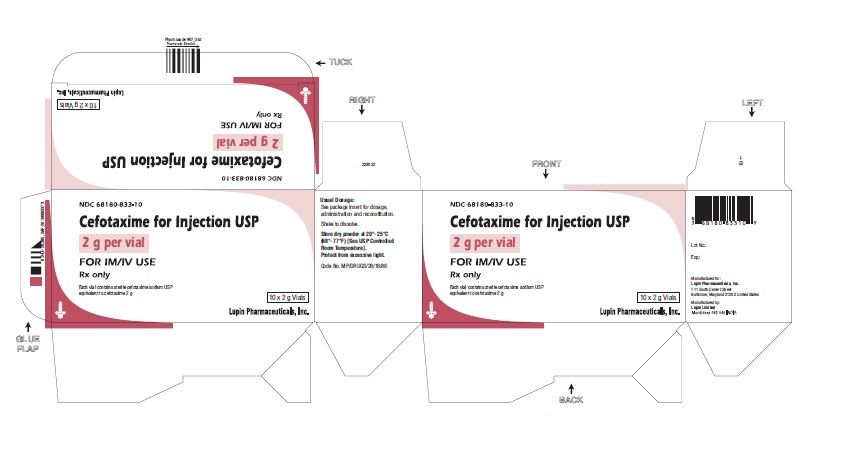
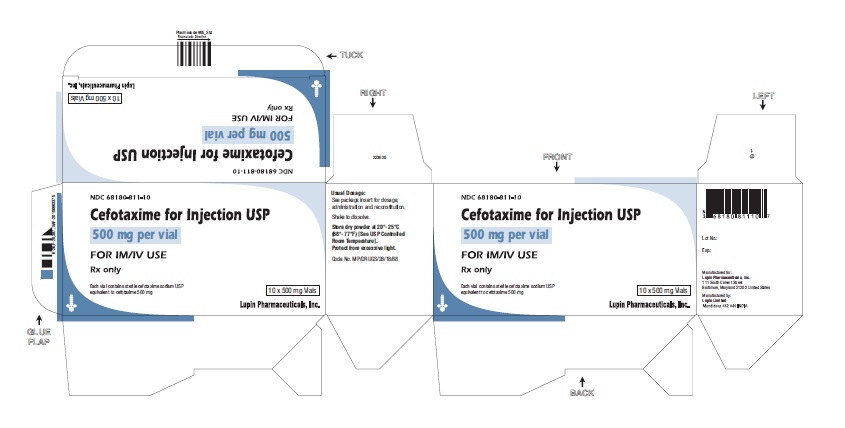
HOW SUPPLIED
Cefotaxime for Injection USP is a dry off-white to pale yellow crystalline powder supplied in vials containing cefotaxime sodium as follows:
500 mg cefotaxime (free acid equivalent) in vials in packages of 10 (NDC 68180-811-10).
1 g cefotaxime (free acid equivalent) in vials in packages of 10 (NDC 68180-822-10), packages of 25 (NDC 68180-822-25), packages of 50 (NDC 68180-822-50).
2 g cefotaxime (free acid equivalent) in vials in packages of 10 (NDC 68180-833-10), packages of 25 (NDC 68180-833-25), packages of 50 (NDC 68180-833-50).
NOTE:
Cefotaxime for injection USP in the dry state should be stored at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. The dry material as well as solutions tend to darken depending on storage conditions and should be protected from elevated temperatures and excessive light.
REFERENCES
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically; Approved Standard - Tenth Edition. CLSI document M07-A10, Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, 2015.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobials Susceptibility Tests; Twenty-Fifth Informational Supplement. CLSI document M100-S25, Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, 2015.
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard - Twelfth Edition. CLSI document M02-A12, Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, 2015.
- Clinical and Laboratory Standards Institute (CLSI). Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria; Approved Standard - Eighth Edition. CLSI document M11-A8, Clinical and Laboratory Standards Institute, 950 West Valley Road, Suite 2500, Wayne, Pennsylvania 19087, USA, 2012.
- Cockcroft, D.W. and Gault, M.H.: Prediction of Creatinine Clearance from Serum Creatinine, Nephron 16:31-41, 1976.
®The brands listed are trademarks of their respective owners and are not trademarks of Lupin Pharmaceuticals, Inc. The makers of these brands are not affiliated with and do not endorse Lupin Pharmaceuticals, Inc. or its products.
Manufactured for:
Lupin Pharmaceuticals, Inc.
111 South Calvert Street
Baltimore, Maryland 21202
United States
Manufactured by:
Lupin Limited
Mandideep 462 046
INDIA
Revised: July 2015 ID#: 223023
| CEFOTAXIME
cefotaxime injection, powder, for solution |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| CEFOTAXIME
cefotaxime injection, powder, for solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| CEFOTAXIME
cefotaxime injection, powder, for solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Lupin Pharmaceuticals, Inc. (089153071) |
| Registrant - LUPIN LIMITED (675923163) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LUPIN LIMITED | 725504448 | MANUFACTURE(68180-811, 68180-822, 68180-833) , PACK(68180-811, 68180-822, 68180-833) | |