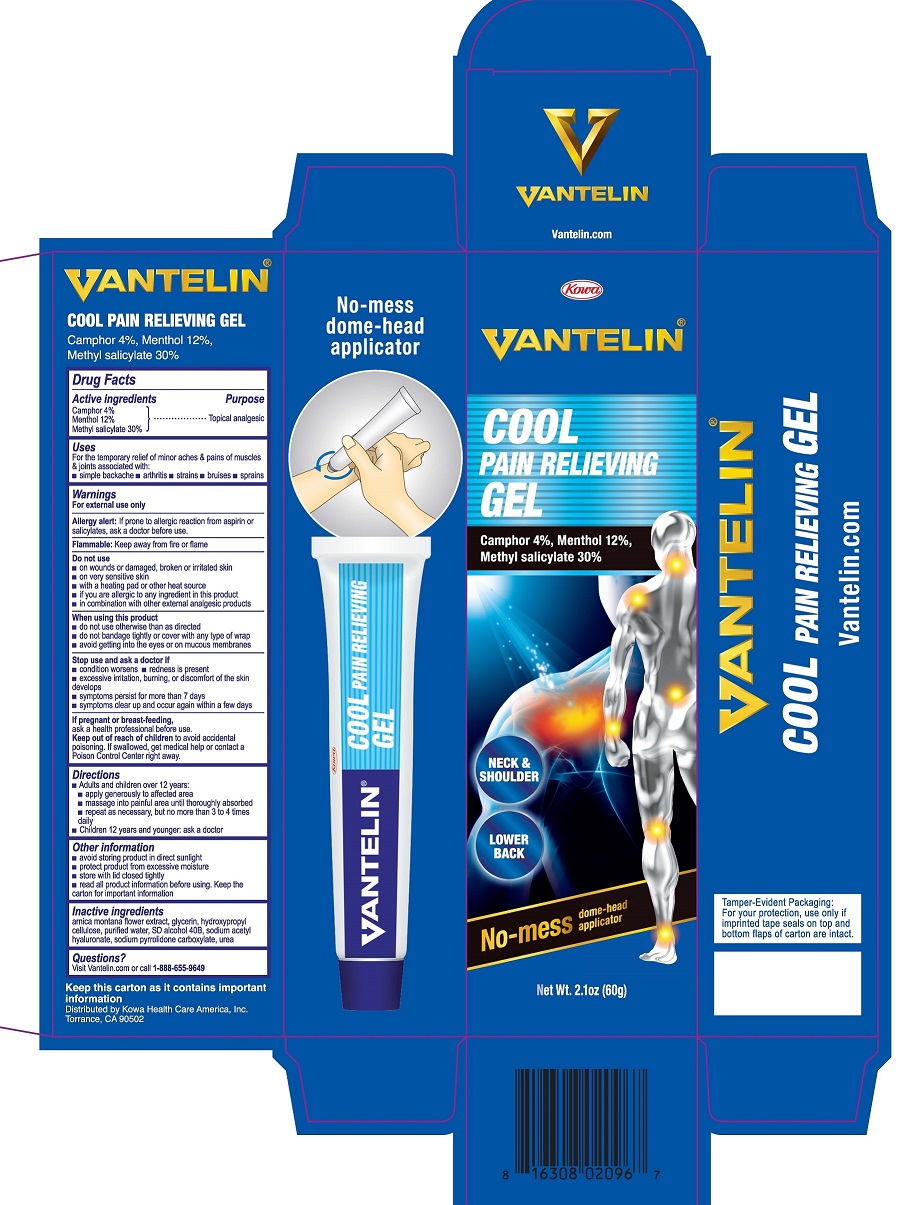
VANTELIN COOL PAIN RELIEVING- camphor, menthol, methyl salicylate gel
Kowa Health Care America, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
VANTELIN COOL PAIN RELIEVING GEL
Uses
For the temporary relief of minor aches & pains of muscles & joints associated with:
- simple backache
- arthritis
- strains
- bruises
- sprains
Warnings
For external use only
Allergy alert: If prone to allergic reaction from aspirin or salicylates, ask a doctor before use.
Flammable: Keep away from fire or flame
Do not use
- on wounds or damaged, broken or irritated skin
- on very sensitive skin
- with a heating pad or other heat source
- if you are allergic to any ingredient in this product
- in combination with other external analgesic products
When using this product
- do not use otherwise than as directed
- do not bandage tightly or cover with any type of wrap
- avoid getting into the eyes or on mucous membranes
Directions
- Adults and children over 12 years:
• apply generously to affected area
• massage into painful area until thoroughly absorbed
• repeat as necessary, but no more than 3 to 4 times daily - Children 12 years and younger: ask a doctor
Other information
- avoid storing product in direct sunlight
- protect product from excessive moisture
- store with lid closed tightly
- read all product information before using. Keep the carton for important information
| VANTELIN COOL PAIN RELIEVING
camphor, menthol, methyl salicylate gel |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Kowa Health Care America, Inc. (078740352) |
Revised: 10/2018
Document Id: 78ef8760-e4a7-57d8-e053-2991aa0af7a5
Set id: 4eb1da45-a7a4-4aba-961a-35db43a6fcf6
Version: 2
Effective Time: 20181023
Kowa Health Care America, Inc.