AZO- urinary pain relief tablet
i-Health, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
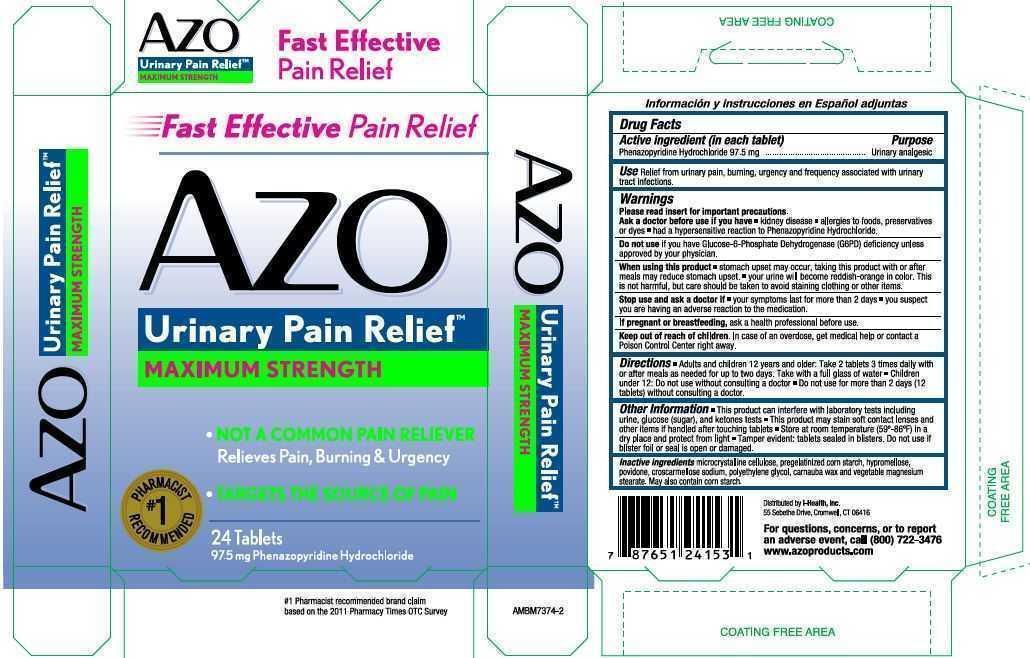
AZO Urinary Pain Relief Maximum Strength
Use: Relief from urinary pain, burning, urgency and frequency associated with urinary tract infections.
Warnings:
Please read insert for important precautions.
Ask a doctor before use if you have
- kidney disease
- allergies to foods, preservatives or dyes
- had a hypersensitive reaction to Phenazopyridine Hydrochloride.
Do not use if you have Glucose-6-Phosphate Dehydrogenase (G6PD) deficiency unless approved by your physician.
When using this product
- stomach upset may occur, taking this product with or after meals may reduce stomach upset.
- your urine will become reddish-orange in color. This is not harmful, but care should be taken to avoid staining clothing or other items.
Stop use and ask doctor if
- your symptoms last for more than 2 days
- you suspect you are having an adverse reaction to the medication.
Directions
- Adults and children 12 years or older: Take 2 tablets 3 times daily with or after meals as needed for up to two days. Take with a full glass of water.
- Children under 12: Do not use without consulting a doctor
- Do not use for more than 2 days (12 tablets) without consulting a doctor.
Other Inf ormation
- This product can interfere with laboratory tests including urine, glucose (sugar), and ketones tests
- This product may stain soft contact lenses and other items if handled after touching tablets
- Store at room temperature (59-86 F) in a dry place and protect from light
- Tamper evident: tablets sealed in blisters. Do not use if blister foil or seal is open or damaged.
- Carcinogenesis: Long-term administration of Phenazopyridine Hydrochloride has induced neoplasia in rats (large intestine) and mice (liver). Although no association between Phenazopyridine Hcl and human neoplasia has been reported, adequate epidemiological studies along these lines have not been conducted.
Inactive ingredients microcrystalline cellulose, pregelatinized corn starch, hypromellose, povidine, croscarmellose sodium, polyethylene glycol, carnauba wax and vegetable magnesium stearate. May also contain corn starch.
| AZO
urinary pain relief tablet |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| Labeler - i-Health, Inc. (061427694) |