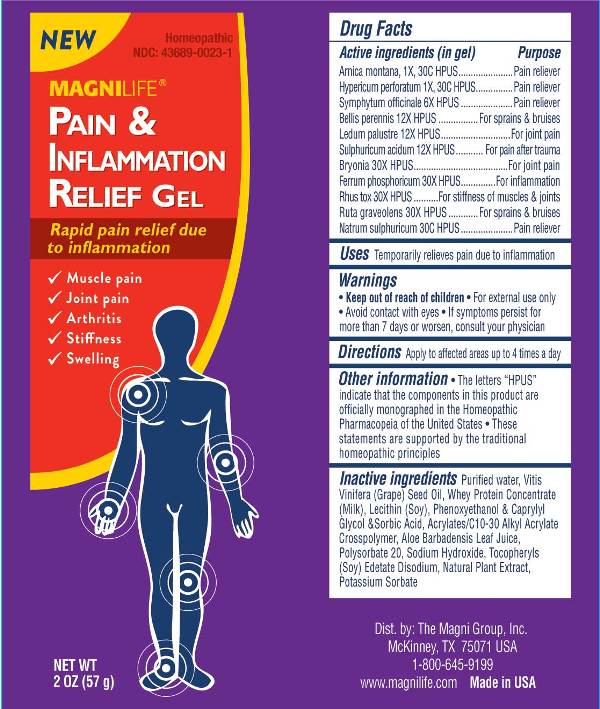
PAIN AND INFLAMMATION- arnica montana, hypericum perforatum, symphytum officinale, bellis perennis, ledum palustre, sulphuricum acidum, bryonia, ferrum phosphoricum, rhus tox, ruta graveolens, natrum sulphuricum gel
The Magni Company
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
Drug Facts:
ACTIVE INGREDIENTS:
Arnica Montana 1X, 30C, Hypericum Perforatum 1X, 30C, Symphytum Officinale 6X, Bellis Perennis 12X, Ledum Palustre 12X, Sulphuricum Acidum 12X, Bryonia 30X, Ferrum Phosphoricum 30X, Rhus Tox 30X, Ruta Graveolens 30X, Natrum Sulphuricum 30C
WARNINGS:
• Keep out of reach of children. • For external use only.
• Avoid contact with eyes. • If symptoms persist for
more than 7 days or worsen, consult your physician.
OTHER INFORMATION:
The letters "HPUS" indicate that the components in this product are officially monographed
in the Homeopathic Pharmacopeia of the United States.
These statements are supported by the traditional homeopathic principles.
INACTIVE INGREDIENTS:
Purified Water, Vitis Vinifera (Grape) Seed Oil, Whey Protein Concentrate (Milk), Lecithin (Soy), Phenoxyethanol & Caprylyl Glycol & Sorbic Acid, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aloe Barbadensis Leaf Juice, Polysorbate 20, Sodium Hydroxide, Tocopheryls (Soy), Edetate Disodium, Natural Plant Extract, Potassium Sorbate
| PAIN AND INFLAMMATION
arnica montana, hypericum perforatum, symphytum officinale, bellis perennis, ledum palustre, sulphuricum acidum, bryonia, ferrum phosphoricum, rhus tox, ruta graveolens, natrum sulphuricum gel |
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||
| Labeler - The Magni Company (113501902) |
| Registrant - Apotheca Company (844330915) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Apotheca Company | 844330915 | manufacture(43689-0023) , api manufacture(43689-0023) , label(43689-0023) , pack(43689-0023) | |