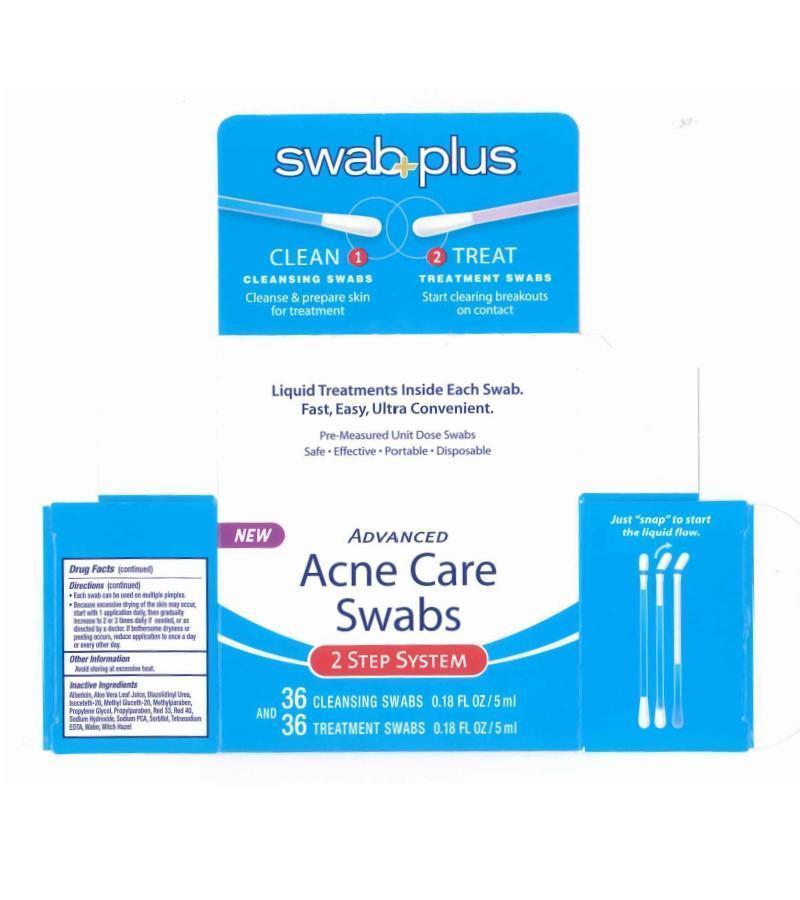
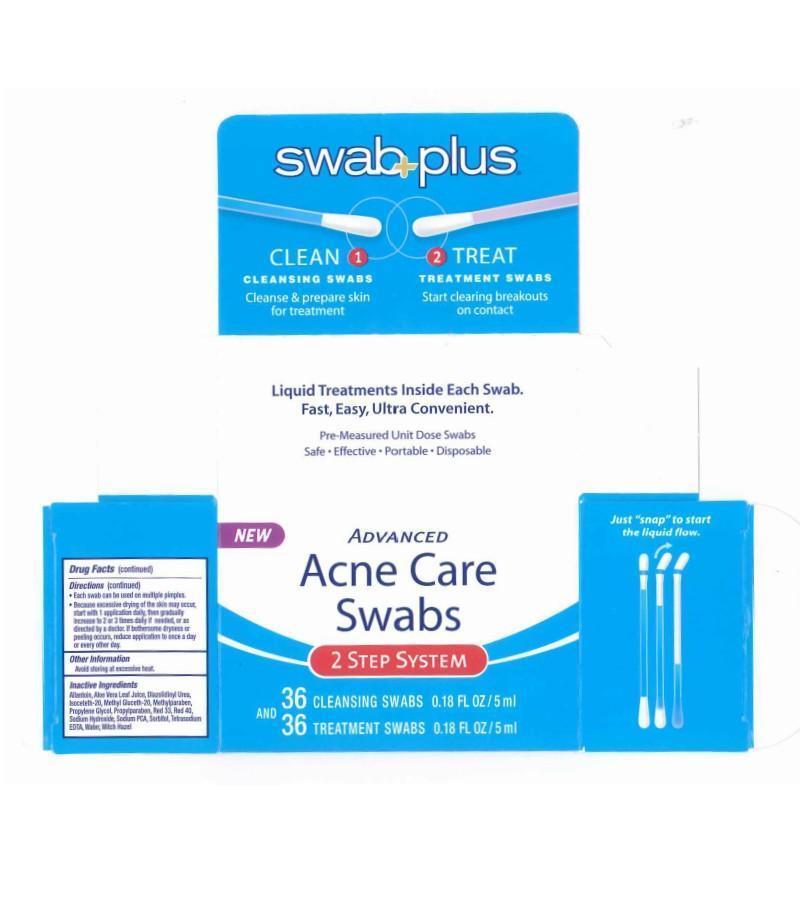
Label: ADVANCE ACNE CARE- salicylic acid, silver kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 65734-740-00, 65734-741-00, 65734-741-24, 65734-742-00, view more65734-742-24 - Packager: Swabplus Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 23, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug FactsActive Ingredients
- Purpose
-
Uses
When using this product. Avoid contact with eyes. If contact occures. flush throughly with water. Using other topical acne medications at teh same time or immediately following use this product may increase dryness or irrtation of the skin. If this occures, only one medication shoukld be used unless directed by a doctor. Do not insert in teh canal.
- Keep out of reach of children
-
Directions
- Do not use if label seal is broken prior to purchase.Keep swabs in original container when not in use. Treatment Swabs have a red color band.
- Use Cleansinh swab to cleanse the skin throughly before applying medication.
- Hold the swab vertically, with the color ring band tip upwards.
- Bend the tip at the color band to one side until it snaps. Medicine will flow to theother end.
- Cover the entire affected area with a thin layer 1 to 3 times daily. Discard swab after use.
- Each swab can be used on multipe pimples.
- Because excesssive drying of the skin may occur start with1 application daiky then gradually increase to 2 or 3 times daily if needed, or as direcvted by a doctor. If bothersome dryness or peeling occurs, reduce application to once a day or every othr day.
- Warnings
- Other information
- Inactive Ingredients
- Package carton
-
INGREDIENTS AND APPEARANCE
ADVANCE ACNE CARE
salicylic acid, silver kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65734-740 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65734-740-00 1 in 1 CARTON Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 13 APPLICATOR 2 mL Part 2 13 APPLICATOR 2 mL Part 1 of 2 ACNE CARE SWAB STEP 1 CLEAN
silver solutionProduct Information Item Code (Source) NDC:65734-741 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SILVER (UNII: 3M4G523W1G) (SILVER - UNII:3M4G523W1G) SILVER 0.1 mg in 10 mL Inactive Ingredients Ingredient Name Strength SODIUM LAURYL SULFATE (UNII: 368GB5141J) CITRIC ACID ACETATE (UNII: DSO12WL7AU) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65734-741-24 24 in 1 PACKAGE 1 NDC:65734-741-00 0.15 mL in 1 APPLICATOR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/07/2008 Part 2 of 2 ACNE CARE SWAB STEP 2 TREAT
salicylic acid solutionProduct Information Item Code (Source) NDC:65734-742 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALLANTOIN (UNII: 344S277G0Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) ISOCETETH-20 (UNII: O020065R7Z) METHYL GLUCETH-20 (UNII: J3QD0LD11P) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM HYDROXIDE (UNII: 55X04QC32I) SODIUM (UNII: 9NEZ333N27) SORBITAN (UNII: 6O92ICV9RU) WATER (UNII: 059QF0KO0R) WITCH HAZEL (UNII: 101I4J0U34) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65734-742-24 24 in 1 PACKAGE 1 NDC:65734-742-00 0.15 mL in 1 APPLICATOR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 08/07/2008 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 08/07/2008 Labeler - Swabplus Inc. (876441549) Registrant - Swabplus Inc. (876441549) Establishment Name Address ID/FEI Business Operations Swabplus Inc. 876441549 repack(65734-740) , manufacture(65734-741, 65734-742)