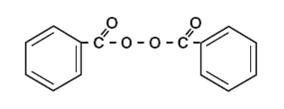BENZOYL PEROXIDE- benzoyl peroxide liquid
Perrigo New York Inc
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Benzoyl Peroxide Wash 2.5%, 5% and 10%
DESCRIPTION
Benzoyl Peroxide Wash 2.5%, 5% and 10% are topical preparations containing benzoyl peroxide as the active ingredient.
Benzoyl Peroxide Wash 2.5%, 5% and 10% each contain: 2.5%, 5% and 10% benzoyl peroxide, respectively, in a lathering base formulated with acrylates copolymer, carbomer homopolymer type C, edetate disodium, glycerin, imidurea, purified water, sodium hydroxide, sodium C14-16 olefin sulfonate. May contain citric acid to adjust pH.
The structural formula of benzoyl peroxide is:
CLINICAL PHARMACOLOGY
The exact method of action of benzoyl peroxide in acne vulgaris is not known. Benzoyl peroxide is an antibacterial agent with demonstrated activity against Propionibacterium acnes. This action, combined with the mild keratolytic effect of benzoyl peroxide is believed to be responsible for its usefulness in acne.
Benzoyl peroxide is absorbed by the skin where it is metabolized to benzoic acid and excreted as benzoate in the urine.
INDICATIONS AND USAGE
Benzoyl Peroxide Wash 2.5%, 5% and 10% are indicated for use in the topical treatment of mild to moderate acne vulgaris. Benzoyl Peroxide Wash 2.5%, 5% and 10% may be used as an adjunct in acne treatment regimens including antibiotics, retinoic acid products, and sulfur/salicylic acid containing preparations.
CONTRAINDICATIONS
Benzoyl Peroxide Wash 2.5%, 5% and 10% should not be used in patients who have shown hypersensitivity to benzoyl peroxide or to any of the other ingredients in the products.
PRECAUTIONS
General
For external use only. Avoid contact with eyes and mucous membranes. AVOID CONTACT WITH HAIR, FABRICS OR CARPETING AS BENZOYL PEROXIDE WILL CAUSE BLEACHING.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Based upon all available evidence, benzoyl peroxide is not considered to be a carcinogen. However, data from a study using mice known to be highly susceptible to cancer suggest that benzoyl peroxide acts as a tumor promoter. The clinical significance of the findings is not known.
Pregnancy
Teratogenic Effects
Pregnancy category C
Animal reproduction studies have not been conducted with benzoyl peroxide. It is also not known whether benzoyl peroxide can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Benzoyl peroxide should be given to a pregnant woman only if clearly needed.
ADVERSE REACTIONS
Contact sensitization reactions are associated with the use of topical benzoyl peroxide products and may be expected to occur in 10 to 25 of 1000 patients. The most frequent adverse reactions associated with benzoyl peroxide use are excessive erythema and peeling which may be expected to occur in 5 of 100 patients. Excessive erythema and peeling most frequently appear during the initial phase of drug use and may normally be controlled by reducing frequency of use.
DOSAGE AND ADMINISTRATION
Shake well before using. It is recommended that therapy be initiated with Benzoyl Peroxide Wash 2.5%, washing the affected areas once a day during the first week, and twice a day thereafter as tolerated. Wet skin areas to be treated; apply Benzoyl Peroxide Wash, work to a full lather, rinse thoroughly and pat dry. Frequency of use should be adjusted to obtain the desired clinical response. Therapy with Benzoyl Peroxide Wash 5% or 10% may be initiated in patients who demonstrate accommodation to Benzoyl Peroxide Wash 2.5%.
Clinically visible improvement will normally occur by the third week of therapy. Maximum lesion reduction may be expected after approximately eight to twelve weeks of drug use. Continuing use of the drug is normally required to maintain a satisfactory clinical response.
HOW SUPPLIED
Benzoyl Peroxide Wash 2.5%, 5% and 10% are available as follows:
Benzoyl Peroxide Wash 2.5%
8 oz plastic bottle (NDC 45802-907-34)
Benzoyl Peroxide Wash 5%
4 oz plastic bottle (NDC 45802-913-26)
5 oz plastic bottle (NDC 45802-913-01)
8 oz plastic bottle (NDC 45802-913-34)
Benzoyl Peroxide Wash 10%
5 oz plastic bottle (NDC 45802-918-01)
8 oz plastic bottle (NDC 45802-918-34)
Manufactured by
Stiefel Laboratories, Inc.
Research Triangle Park, NC 27709
Distributed By
Perrigo®
Allegan, MI 49010 • www.perrigo.com
Rev. 12/10
305263
: 0V500 RC J3
| BENZOYL PEROXIDE
benzoyl peroxide liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Perrigo New York Inc (078846912) |
| Registrant - L. Perrigo Company (006013346) |