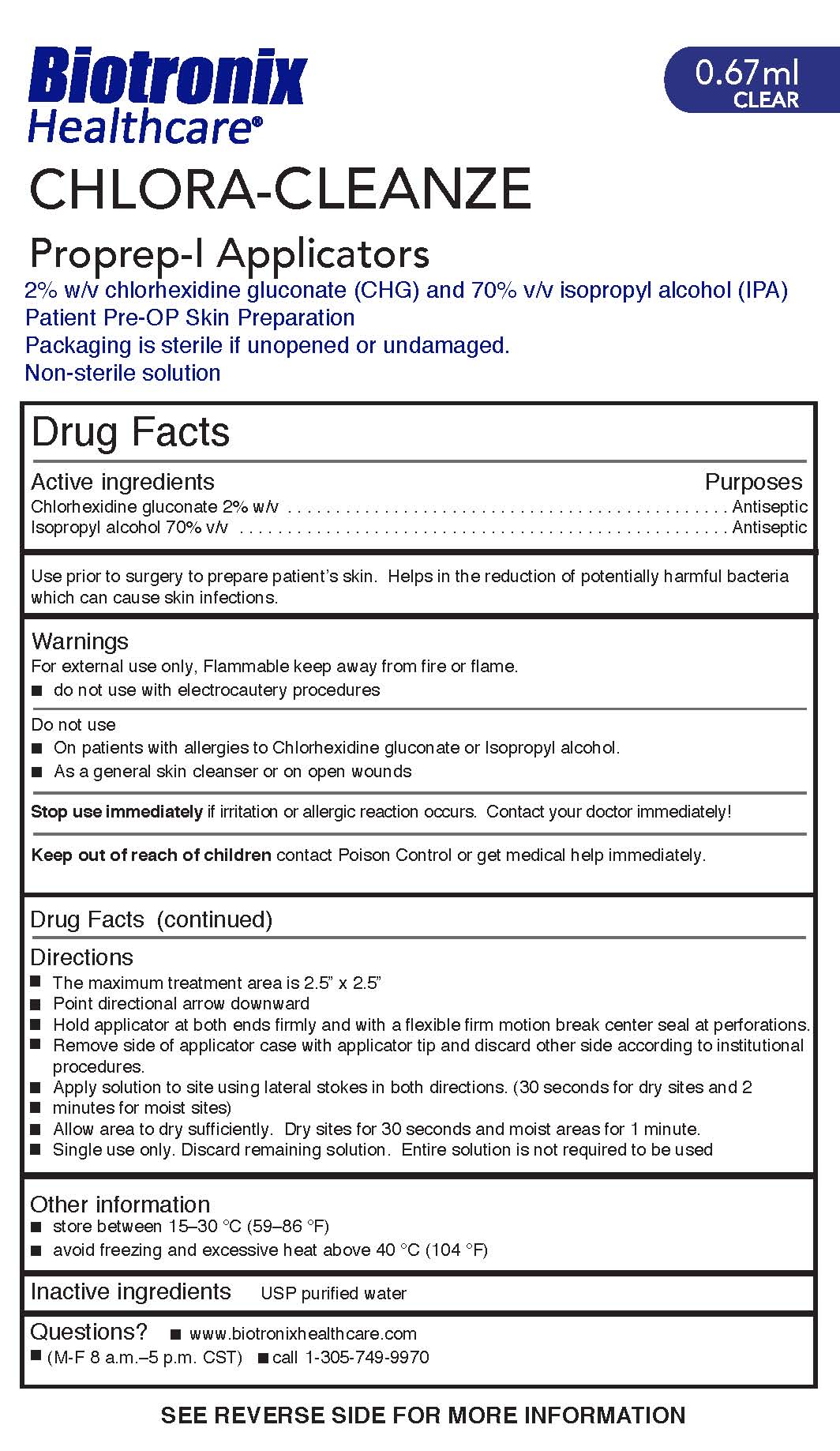
Label: CHLORA-CLEANZE- chlorhexidine gluconate and isopropyl alcohol swab
-
Contains inactivated NDC Code(s)
NDC Code(s): 71389-001-01 - Packager: Biotronix Healthcare Industries, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 5, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- STOP USE
-
DOSAGE & ADMINISTRATION
Directions
- The maximum treatment area is 2.5” x 2.5”
- Point directional arrow downward
- Hold applicator at both ends firmly and with a flexible firm motion break center seal at perforations.
- Remove side of applicator case with applicator tip and discard other side according to institutional
procedures.
- Apply solution to site using lateral stokes in both directions. (30 seconds for dry sites and 2
minutes for moist sites)
- Allow area to dry sufficiently. Dry sites for 30 seconds and moist areas for 1 minute.
- Single use only. Discard remaining solution. Entire solution is not required to be used
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- STORAGE AND HANDLING
- KEEP OUT OF REACH OF CHILDREN
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CHLORA-CLEANZE
chlorhexidine gluconate and isopropyl alcohol swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71389-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE GLUCONATE 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71389-001-01 0.67 mL in 1 POUCH; Type 0: Not a Combination Product 04/18/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 04/18/2017 Labeler - Biotronix Healthcare Industries, Inc. (065762392) Registrant - US MED PHARM SUPPLIES, INC. (831533109) Establishment Name Address ID/FEI Business Operations Longood Medicine (Beijing) Co., Ltd. 529553064 manufacture(71389-001)