CAPRELSA- vandetanib tablet
AstraZeneca Pharmaceuticals LP
----------
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use CAPRELSA safely and effectively. See full prescribing information for CAPRELSA.
CAPRELSA® (vandetanib) Tablets for Oral use Initial U.S. Approval: 2011 WARNING: QT PROLONGATION, TORSADES DE POINTES, AND SUDDEN DEATHSee full prescribing information for complete boxed warning.CAPRELSA can prolong the QT interval. Torsades de pointes and sudden death have occurred in patients receiving CAPRELSA. Do not use CAPRELSA in patients with hypocalcemia, hypokalemia, hypomagnesemia, or long QT syndrome. Correct hypocalcemia, hypokalemia and/or hypomagnesemia prior to CAPRELSA administration. Monitor electrolytes periodically. Avoid drugs known to prolong the QT interval. Only prescribers and pharmacies certified with the restricted distribution program are able to prescribe and dispense CAPRELSA (5.1, 5.15). RECENT MAJOR CHANGES
Warnings and Precautions (5.2) 3/2016 INDICATIONS AND USAGECAPRELSA is a kinase inhibitor indicated for the treatment of symptomatic or progressive medullary thyroid cancer in patients with unresectable locally advanced or metastatic disease. (1) Use of CAPRELSA in patients with indolent, asymptomatic or slowly progressing disease only after careful consideration of the treatment related risks of CAPRELSA. (1) DOSAGE AND ADMINISTRATIONDOSAGE FORMS AND STRENGTHS100 mg and 300 mg tablets (3) CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONSThe most common adverse drug reactions (>20%) seen with CAPRELSA and with a between-arm difference of ≥5 % have been diarrhea/colitis, rash, acneiform dermatitis, hypertension, nausea, headache, upper respiratory tract infections, decreased appetite and abdominal pain. To report SUSPECTED ADVERSE REACTIONS, contact AstraZeneca at 1–800–236–9933 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONSSee 17 for PATIENT COUNSELING INFORMATION and Medication Guide. Revised: 3/2016 |
FULL PRESCRIBING INFORMATION
WARNING: QT PROLONGATION, TORSADES DE POINTES, AND SUDDEN DEATH
CAPRELSAcan prolong the QT interval. Torsades de pointes and sudden death have occurred in patients receiving CAPRELSA. Do not use CAPRELSA in patients with hypocalcemia, hypokalemia, hypomagnesemia, or long QT syndrome. Correct hypocalcemia, hypokalemia and/or hypomagnesemia prior to CAPRELSA administration. Monitor electrolytes periodically. Avoid drugs known to prolong the QT interval. Only prescribers and pharmacies certified with the restricted distribution program are able to prescribe and dispense CAPRELSA [seeWarnings and Precautions (5.1), (5.15)].
1. INDICATIONS AND USAGE
CAPRELSA is indicated for the treatment of symptomatic or progressive medullary thyroid cancer in patients with unresectable locally advanced or metastatic disease.
Use CAPRELSA in patients with indolent, asymptomatic or slowly progressing disease only after careful consideration of the treatment related risks of CAPRELSA.
2. DOSAGE AND ADMINISTRATION
The recommended dose of CAPRELSA is 300 mg taken orally once daily until disease progression or unacceptable toxicity occurs.
CAPRELSA may be taken with or without food.
Do not take a missed dose within 12 hours of the next dose.
Do not crush CAPRELSA tablets. The tablets can be dispersed in 2 ounces of water by stirring for approximately 10 minutes (will not completely dissolve). Do not use other liquids for dispersion. Swallow immediately after dispersion. Mix any remaining residue with 4 additional ounces of water and swallow.
The dispersion can also be administered through nasogastric or gastrostomy tubes.
2.1 Dosage Adjustment
For adverse reactions
The 300 mg daily dose can be reduced to 200 mg (two 100 mg tablets) and then to 100 mg for Common Terminology Criteria for Adverse Events (CTCAE) Grade 3 or greater toxicities.
Interrupt CAPRELSA for the following:
- •
- Corrected QT interval, Fridericia (QTcF) greater than 500 ms: Resume at a reduced dose when the QTcF returns to less than 450 ms.
- •
- CTCAE Grade 3 or greater toxicity: Resume at a reduced dose when the toxicity resolves or improves to CTCAE Grade 1.
For recurrent toxicities, reduce the dose of CAPRELSA to 100 mg after resolution or improvement to CTCAE Grade 1 severity, if continued treatment is warranted.
Because of the 19-day half-life, adverse reactions including a prolonged QT interval may not resolve quickly. Monitor appropriately [seeWarnings and Precautions (5.1),(5.2),(5.3),(5.4),(5.5),(5.6),(5.7), and (5.9)].
For patients with renal impairment
Reduce the starting dose to 200 mg in patients with moderate (creatinine clearance ≥30 to <50 mL/min) and severe (creatinine clearance <30 mL/min) renal impairment [seeWarnings and Precautions (5.12)andUse in Specific Populations (8.6)].
For patients with hepatic impairment
CAPRELSA is not recommended for use in patients with moderate and severe hepatic impairment [seeUse in Specific Populations (8.7)].
3. DOSAGE FORMS & STRENGTHS
CAPRELSA 100-mg tablets are white, round, biconvex, film-coated, and intagliated with ‘Z 100’ on one side and plain on the reverse side.
CAPRELSA 300-mg tablets are white, oval, biconvex, film-coated, and intagliated with ‘Z 300’ on one side and plain on the reverse side.
5. WARNINGS AND PRECAUTIONS
5.1 QT Prolongation and Torsades de Pointes
CAPRELSA can prolong the QT interval in a concentration-dependent manner [seeClinical Pharmacology (12.2)]. Torsades de pointes, ventricular tachycardia and sudden deaths have occurred in patients treated with CAPRELSA.
Do not start CAPRELSA treatment in patients whose QTcF interval is greater than 450 ms. Do not administer CAPRELSA to patients who have a history of Torsades de pointes, congenital long QT syndrome, bradyarrhythmias or uncompensated heart failure. CAPRELSA has not been studied in patients with ventricular arrhythmias or recent myocardial infarction. Vandetanib exposure is increased in patients with impaired renal function. Reduce the starting dose to 200 mg in patients with moderate to severe renal impairment and monitor QT interval frequently.
Obtain an ECG and serum potassium, calcium, magnesium and TSH at baseline, 2-4 weeks and 8-12 weeks after starting treatment with CAPRELSA, and every 3 months thereafter. Monitor electrolytes and ECGs more frequently in patients who experience diarrhea. Following any dose reduction for QT prolongation or any dose interruption greater than 2 weeks, conduct QT assessments as described above. Maintain serum potassium levels of 4 mEq/L or higher (within normal range) and maintain serum magnesium and calcium levels within normal ranges to reduce the risk of QT prolongation.
Avoid using CAPRELSA with drugs known to prolong the QT interval [seeWarnings and Precautions (5.11)andDrug Interactions (7.4)]. If such drugs are given to patients already receiving CAPRELSA and no alternative therapy exists, perform ECG monitoring of the QT interval more frequently.
Stop CAPRELSA in patients who develop a QTcF greater than 500 ms until the QTcF returns to less than 450 ms. Dosing of CAPRELSA can then be resumed at a reduced dose [seeDosage and Administration (2.1)].
5.2 Skin Reactions
Severe skin reactions (including Stevens-Johnson syndrome and Toxic Epidermal Necrolysis), some leading to death, have occurred in patients treated with CAPRELSA. For severe skin reactions, referral of the patient to seek urgent medical advice is recommended. Systemic therapies e.g., steroids, may be appropriate in such cases and permanent discontinuation of CAPRELSA is recommended[see Dosage and Administration (2.1)].
Photosensitivity reactions can occur during CAPRELSA treatment and up to 4 months after treatment discontinuation.
5.3 Interstitial Lung Disease
Interstitial Lung Disease (ILD) or pneumonitis, including fatalities, has occurred in patients treated with CAPRELSA. Consider a diagnosis of ILD in patients presenting with non-specific respiratory signs and symptoms.
Interrupt CAPRELSA for acute or worsening pulmonary symptoms. Discontinue CAPRELSA if ILD is confirmed.
5.4 Ischemic Cerebrovascular Events
Ischemic cerebrovascular events, including fatalities, occurred in patients treated with CAPRELSA. In the randomized medullary thyroid cancer (MTC) study, ischemic cerebrovascular events occurred more frequently with CAPRELSA compared to placebo (1.3% compared to 0%). The safety of resumption of CAPRELSA therapy after resolution of an ischemic cerebrovascular event has not been studied. Discontinue CAPRELSA in patients who experience a severe ischemic cerebrovascular event.
5.5 Hemorrhage
Serious hemorrhagic events, including fatalities, occurred in patients treated with CAPRELSA. Do not administer CAPRELSA to patients with a recent history of hemoptysis of ≥1/2 teaspoon of red blood. Discontinue CAPRELSA in patients with severe hemorrhage.
5.6 Heart Failure
Heart failure, including fatalities, occurred in patients treated with CAPRELSA. Monitor for signs and symptoms of heart failure. Consider discontinuation of CAPRELSA in patients with heart failure. Heart failure may not be reversible upon stopping CAPRELSA.
5.7 Diarrhea
Diarrhea of Grade 3 or greater severity occurred in 11% of patients receiving CAPRELSA in the randomized MTC study. If diarrhea occurs, carefully monitor serum electrolytes and ECGs to reduce the risk and enable early detection of QT prolongation resulting from dehydration [seeWarnings and Precautions (5.1)]. Interrupt CAPRELSA for severe diarrhea. Upon improvement, resume CAPRELSA at a reduced dose [seeDosage and Administration (2.1)].
5.8 Hypothyroidism
In the randomized MTC study in which 90% of the patients enrolled had prior thyroidectomy, increased dosing of thyroid replacement therapy was required in 49% of CAPRELSA-treated patients compared to 17% of placebo-treated patients. Obtain Thyroid-stimulating hormone (TSH) at baseline, at 2-4 weeks and 8-12 weeks after starting treatment with CAPRELSA, and every 3 months thereafter. If signs or symptoms of hypothyroidism occur, examine thyroid hormone levels and adjust thyroid replacement therapy accordingly.
5.9 Hypertension
Hypertension, including hypertensive crisis, has occurred in patients treated with CAPRELSA. Monitor all patients for hypertension. Dose reduction or interruption for hypertension may be necessary. If hypertension cannot be controlled, do not resume CAPRELSA [seeDosage and Administration, (2.1)].
5.10 Reversible Posterior Leukoencephalopathy Syndrome
Reversible posterior leukoencephalopathy syndrome (RPLS), a syndrome of subcortical vasogenic edema diagnosed by an MRI of the brain, has occurred in patients treated with CAPRELSA. Consider this syndrome in any patient presenting with seizures, headache, visual disturbances, confusion or altered mental function. In clinical studies, three of four patients who developed RPLS while taking CAPRELSA also had hypertension. Discontinue CAPRELSA treatment in patients with RPLS.
5.11 Drug Interactions
Avoid administration of CAPRELSA with anti-arrhythmic drugs (including but not limited to amiodarone, disopyramide, procainamide, sotalol, dofetilide) and other drugs that may prolong the QT interval (including but not limited to chloroquine, clarithromycin, dolasetron, granisetron, haloperidol, methadone, moxifloxacin, and pimozide) [seeDrug Interactions (7.4)andClinical Pharmacology (12.2)].
5.12 Renal Impairment
Vandetanib exposure is increased in patients with impaired renal function. Reduce the starting dose to 200 mg in patients with moderate to severe renal impairment and monitor the QT interval closely. There is no information available for patients with end-stage renal disease requiring dialysis [seeBoxed Warning, Dosage and Administration (2.1), Use in Specific Populations (8.6)andClinical Pharmacology (12.3)].
5.13 Hepatic Impairment
CAPRELSA is not recommended for use in patients with moderate and severe hepatic impairment, as safety and efficacy have not been established [seeDosage and Administration (2.1)].
5.14 Embryofetal Toxicity
Based on its mechanism of action, CAPRELSA can cause fetal harm when administered to a pregnant woman. In nonclinical studies in rats, vandetanib was embryotoxic, fetotoxic, and teratogenic at exposures equivalent to or lower than those expected at the recommended human dose of 300 mg/day and had adverse effects on female fertility, embryofetal development, and postnatal development of pups.
If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus. Women of childbearing potential should avoid pregnancy. Advise women of childbearing potential that they must use effective contraception during CAPRELSA treatment and for at least four months following the last dose of CAPRELSA [see Use in Specific Populations (8.1), (8.8)].
5.15 CAPRELSA REMS (Risk Evaluation and Mitigation Strategy) Program
Because of the risk of QT prolongation, Torsades de pointes, and sudden death, CAPRELSA is available only through a restricted distribution program called the CAPRELSA REMS Program. Only prescribers and pharmacies certified with the program are able to prescribe and dispense CAPRELSA.
To learn about the specific REMS requirements and to enroll in the CAPRELSA REMS Program, call 1-800-236-9933 or visit www.caprelsarems.com.
6. ADVERSE REACTIONS
The following serious adverse reactions are discussed elsewhere in the label:
- •
- QT Prolongation and Torsades de Pointes [seeBoxed Warning, Warnings and Precautions (5.1)]
- •
- Skin Reactions [seeWarnings and Precautions (5.2)]
- •
- Interstitial Lung Disease [seeWarnings and Precautions (5.3)]
- •
- Ischemic Cerebrovascular Events [seeWarnings and Precautions (5.4)]
- •
- Hemorrhage [seeWarnings and Precautions (5.5)]
- •
- Heart Failure [seeWarnings and Precautions (5.6)]
- •
- Diarrhea [seeWarnings and Precautions (5.7)]
- •
- Hypothyroidism [seeWarnings and Precautions (5.8)]
- •
- Hypertension [seeWarnings and Precautions (5.9)]
- •
- Reversible Posterior Leukoencephalopathy Syndrome [seeWarnings and Precautions (5.10)]
- •
- Embryofetal Toxicity [seeWarnings and Precautions (5.14)]
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Patients with unresectable locally advanced or metastatic medullary thyroid cancer were treated with CAPRELSA 300 mg (n=231) or Placebo (n=99). The population exposed to CAPRELSA was 58% male, 94% white, and had a median age of 50 years. The data described below reflect a median exposure to CAPRELSA for 607 days.
The most commonly reported adverse drug reactions which occurred in >20% of CAPRELSA-treated patients and with a between-arm difference of ≥5% included, in order of decreasing frequency: diarrhea/colitis, rash, acneiform dermatitis, hypertension, nausea, headache, upper respiratory tract infection, decreased appetite, and abdominal pain.
Among CAPRELSA-treated patients, dose interruption occurred in 109 (47%) and dose reduction occurred in 83 (36%). Adverse reactions led to study treatment discontinuation in 28 of 231 patients (12%) receiving CAPRELSA and in 3 of 99 patients (3.0%) receiving placebo. Adverse reactions leading to permanent discontinuation in 2 or more (≥0.9%) patients treated with CAPRELSA were: asthenia (1.7%), rash (1.7%), diarrhea (0.9%), fatigue (0.9%), pyrexia (0.9%), elevated creatinine (0.9%), QT prolongation (0.9%), and hypertension (0.9%).
|
||||
|
System Organ Class
|
CAPRELSA 300 mg N=231 |
Placebo N=99 |
||
|
All Grades (%) |
Grade 3-4 (%) |
All Grades (%) |
Grade 3-4 (%) |
|
|
Gastrointestinal Disorders | ||||
|
57 |
11 |
27 |
2 |
|
33 |
1 |
16 |
0 |
|
21 |
3 |
11 |
0 |
|
15 |
1 |
7 |
0 |
|
11 |
0 |
4 |
0 |
|
9 |
0 |
3 |
0 |
|
Skin and Cutaneous Disorders | ||||
|
53 |
5 |
12 |
0 |
|
35 |
1 |
7 |
0 |
|
15 |
0 |
5 |
0 |
|
13 |
2 |
0 |
0 |
|
11 |
1 |
4 |
0 |
|
9 |
0 |
0 |
0 |
|
8 |
N/A |
0 |
N/A |
|
Vascular Disorders | ||||
|
33 |
9 |
5 |
1 |
|
Nervous System Disorders | ||||
|
26 |
1 |
9 |
0 |
|
8 |
0 |
3 |
0 |
|
General Disorders | ||||
|
24 |
6 |
23 |
1 |
|
Infections | ||||
|
Upper Respiratory Tract Infections# |
23 |
0 |
16 |
0 |
|
Metabolic and Nutritional Disorders | ||||
|
21 |
4 |
12 |
0 |
|
11 |
2 |
3 |
0 |
|
Investigations | ||||
|
14 |
8 |
1 |
1 |
|
Eye Disorders | ||||
|
13 |
0 |
1 |
0 |
|
9 |
0 |
1 |
0 |
|
Renal Disorders | ||||
|
10 |
0 |
2 |
0 |
|
Psychiatric Disorders | ||||
|
10 |
2 |
3 |
0 |
|
Endocrine Disorders | ||||
|
6 |
0 |
0 |
0 |
|
Musculoskeletal Disorders | ||||
|
6 |
0 |
1 |
0 |
Clinically important uncommon adverse drug reactions in patients who received CAPRELSA versus patients who received placebo included pancreatitis (0.4% vs. 0%) and heart failure (0.9% vs. 0%).
Blurred vision was more common in patients who received CAPRELSA versus patients who received placebo for medullary thyroid cancer (9% vs. 1%, respectively). Scheduled slit lamp examinations revealed corneal opacities (vortex keratopathies) in treated patients, which can lead to halos and decreased visual acuity. Perform ophthalmologic examination, including slit lamp examination, in patients who report visual changes.
Class effects
CAPRELSA is an inhibitor of vascular endothelial growth factor receptor (VEGFR) signaling. Inhibition of VEGFR signaling can result in intestinal perforation. Intestinal perforation occurred in 0.4% of CAPRELSA treated patients versus 0% of placebo treated patients.
The incidence of Grade 1-2 bleeding events was 14% in patients receiving CAPRELSA compared with 7% on placebo in the randomized portion of the medullary thyroid cancer (MTC) study.
| Laboratory Abnormalities | CAPRELSA 300 mg
N=231 | Placebo
N=99 |
||
|---|---|---|---|---|
|
||||
|
All Grades (%) |
Grade 3–4 (%) |
All Grades (%) |
Grade 3–4 (%) |
|
|
Chemistries | ||||
|
57 |
6 |
25 |
3 |
|
51 |
2 |
19 |
0 |
|
24 |
0 |
7 |
1 |
|
16 |
0 |
1 |
0 |
|
7 |
<1 |
2 |
0 |
|
Hematologic | ||||
|
10 |
<1 |
5 |
2 |
|
9 |
0 |
3 |
0 |
No patient with a Grade 3-4 ALT elevation had a concomitant increase in bilirubin in the MTC study.
7. DRUG INTERACTIONS
7.1 Effect of CYP3A4 Inducers on CAPRELSA
Rifampicin, a strong CYP3A4 inducer, decreased vandetanib plasma concentrations. Avoid concomitant use of known strong CYP3A4 inducers during CAPRELSA therapy. Avoid concomitant use of St. John’s Wort because it can decrease vandetanib exposure unpredictably [see Clinical Pharmacology (12.3)].
7.2 Effect of CAPRELSA on OCT2 Transporter
CAPRELSA increased plasma concentrations of metformin that is transported by the organic cation transporter type 2 (OCT2). Use caution and closely monitor for toxicities when administering CAPRELSA with drugs that are transported by OCT2 [see Clinical Pharmacology (12.3)].
7.3 Effect of CAPRELSA on Digoxin
CAPRELSA increased plasma concentrations of digoxin. Use caution and closely monitor for toxicities when administering CAPRELSA with digoxin [seeClinical Pharmacology (12.3)].
7.4 Drugs that Prolong the QT Interval
Avoid concomitant use of CAPRELSA with agents that may prolong the QT interval [see Warnings and Precautions (5.11)].
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category D [seeWarnings and Precautions (5.14)]
Risk Summary
Based on its mechanism of action, CAPRELSA can cause fetal harm when administered to a pregnant woman. Vandetanib is embryotoxic, fetotoxic, and teratogenic in rats, at exposures less than or equal to those expected at the recommended human dose of 300 mg/day. If CAPRELSA is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus.
Animal data
When vandetanib was administered to female rats prior to mating and through the first week of pregnancy at a dose of 25 mg/kg/day (approximately equal to the human exposure at the recommended dose based on Cmax), there were increases in pre-implantation loss and post-implantation loss resulting in a reduction in the number of live embryos.
During organogenesis, a vandetanib dose of 25 mg/kg administered to rats caused an increase in post-implantation loss, including occasional total litter loss. At doses greater than 10 mg/kg (approximately 0.4 times the human exposure at the recommended dose by Cmax) treatment with vandetanib resulted in increases in late embryofetal death and decreases in fetal birth weight. A no effect level for malformations was not identified in this study. Administration of vandetanib at doses greater than or equal to 1 mg/kg/day (approximately 0.03 times, the Cmax in patients with cancer at the recommended dose) resulted in dose dependent increases in both malformations of the heart vessels and skeletal variations including delayed ossification of the skull, vertebrae, and sternum, indicating delayed fetal development.
In a rat pre- and post-natal development study, at doses producing mild maternal toxicity (1 and 10 mg/kg/day) during gestation and/or lactation, vandetanib decreased pup survival and/or reduced post-natal pup growth. Reduced post-natal pup growth was associated with a delay in physical development.
8.3 Nursing Mothers
In nonclinical studies, vandetanib was excreted in rat milk and found in plasma of pups following dosing to lactating rats. Vandetanib transfer in breast milk resulted in relatively constant exposure in pups due to the long half-life of the drug. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from CAPRELSA, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.5 Geriatric Use
The MTC study of CAPRELSA did not include sufficient numbers of patients aged 65 years and over to determine whether they respond differently compared to younger patients.
8.6 Renal Impairment
Vandetanib exposure is increased in patients with impaired renal function. Reduce the starting dose to 200 mg in patients with moderate (creatinine clearance ≥30 to <50 mL/min) and severe (creatinine clearance <30 mL/min) renal impairment [seeDosage and Administration (2.1), Warnings and Precautions (5.12)andClinical Pharmacology (12.3)].
8.7 Hepatic Impairment
The pharmacokinetics of CAPRELSA were evaluated after a single dose of 800 mg in subjects with mild (n=8), moderate (n=7), and severe (n=6) hepatic impairment and normal hepatic function (n=5). Subjects with mild (Child-Pugh class A), moderate (Child-Pugh class B), and severe (Child-Pugh class C) hepatic impairment had comparable mean AUC and clearance values to those with normal hepatic function.
There are limited data in patients with liver impairment (serum bilirubin greater than 1.5 times the upper limit of normal). CAPRELSA is not recommended for use in patients with moderate and severe hepatic impairment, as safety and efficacy have not been established [seeDosage and Administration (2.1) andWarnings and Precautions (5.13)].
8.8 Females and Males of Reproductive Potential
Contraception
Females of reproductive potential should avoid pregnancy.
Use effective contraception during treatment and up to 4 months after the last dose of CAPRELSA.
Infertility
There are no data on the effect of CAPRELSA on human fertility. Results from animal studies indicate that vandetanib can impair male and female fertility [seeNonclinical Toxicology (13.1)].
10. OVERDOSAGE
In the event of an overdose, monitor patients closely for QTc prolongation. Because of the 19-day half-life, adverse reactions may not resolve quickly.
11. DESCRIPTION
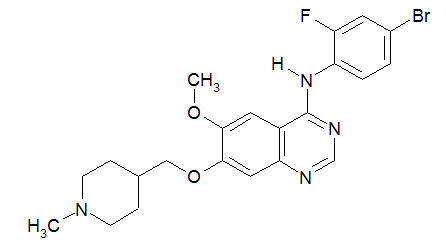
Vandetanib has the chemical name N-(4-bromo-2-fluorophenyl)-6-methoxy-7-[(1-methylpiperidin-4-yl) methoxy]quinazolin-4-amine.
The structural and molecular formulas are:
C22H24BrFN4O2
Vandetanib has a molecular weight of 475.36. Vandetanib exhibits pH-dependent solubility, with increased solubility at lower pH. Vandetanib is practically insoluble in water with a value of 0.008 mg/mL at 25°C (77°F).
CAPRELSA tablets for daily oral administration are available in two dosage strengths containing either 100 mg or 300 mg of vandetanib. The tablet cores contain the following inactive ingredients: calcium hydrogen phosphate dihydrate, microcrystalline cellulose, crospovidone, povidone, and magnesium stearate. The tablet film-coat contains the following inactive ingredients: hypromellose 2910, macrogol 300, and titanium dioxide E171.
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
In vitro studies have shown that vandetanib inhibits the tyrosine kinase activity of the EGFR and VEGFR families, RET, BRK, TIE2, and members of the EPH receptor and Src kinase families. These receptor tyrosine kinases are involved in both normal cellular function and pathologic processes such as oncogenesis, metastasis, tumor angiogenesis, and maintenance of the tumor microenvironment. In addition, the N-desmethyl metabolite of the drug, representing 7 to 17.1% of vandetanib exposure, has similar inhibitory activity to the parent compound for VEGF receptors (KDR and Flt-1) and EGFR.
In vitro, vandetanib inhibited epidermal growth factor (EGF)-stimulated receptor tyrosine kinase phosphorylation in tumor cells and endothelial cells and VEGF-stimulated tyrosine kinase phosphorylation in endothelial cells.
In vivo, vandetanib administration reduced tumor cell-induced angiogenesis, tumor vessel permeability, and inhibited tumor growth and metastasis in mouse models of cancer.
12.2 Pharmacodynamics
Cardiac Electrophysiology
In 231 patients with medullary thyroid cancer randomized to receive CAPRELSA 300 mg once daily in the phase 3 clinical trial. CAPRELSA was associated with sustained plasma concentration-dependent QT prolongation. Based on the exposure-response relationship, the mean (90% CI) QTcF change from baseline (ΔQTcF) was 35 (33-36) ms for the 300 mg dose. The ΔQTcF remained above 30 ms for the duration of the trial (up to 2 years). In addition, 36% of patients experienced greater than 60 ms increase in ΔQTcF and 4.3% of patients had QTcF greater than 500 ms. Cases of Torsades de pointes and sudden death have occurred [seeBoxed Warning and Warnings and Precautions (5.1),(5.11)].
12.3. Pharmacokinetics
A population pharmacokinetic analysis of CAPRELSA was conducted in 231 patients with MTC following oral administration of 300 mg daily doses. The pharmacokinetics of CAPRELSA at the 300 mg dose in MTC patients are characterized by a mean clearance of approximately 13.2 L/h, a mean volume of distribution of approximately 7450 L, and a median plasma half-life of 19 days.
Absorption
Following oral administration of CAPRELSA, absorption is slow with peak plasma concentrations typically achieved at a median of 6 hours, range 4-10 hours, after dosing. Vandetanib accumulates approximately 8-fold on multiple dosing with steady state achieved in approximately 3 months.
Exposure to vandetanib is unaffected by food.
Distribution
Vandetanib binds to human serum albumin and α1-acid-glycoprotein with in vitro protein binding being approximately 90%. In ex vivo plasma samples from colorectal cancer patients at steady state exposure after 300 mg once daily, the mean percentage protein binding was 94%.
Metabolism
Following oral dosing of 14C-vandetanib, unchanged vandetanib and metabolites vandetanib N-oxide and N-desmethyl vandetanib were detected in plasma, urine and feces. A glucuronide conjugate was seen as a minor metabolite in excreta only. N-desmethyl-vandetanib is primarily produced by CYP3A4 and vandetanib-N-oxide by flavin–containing monooxygenase enzymes FMO1 and FMO3. N-desmethyl-vandetanib and vandetanib-N-oxide circulate at concentrations of approximately 7-17% and 1.4-2.2%, respectively, of those of vandetanib.
Excretion
Within a 21-day collection period after a single dose of 14C-vandetanib, approximately 69% was recovered with 44% in feces and 25% in urine. Excretion of the dose was slow and further excretion beyond 21 days would be expected based on the plasma half-life.
Vandetanib was not a substrate of hOCT2 expressed in HEK293 cells. Vandetanib inhibits the uptake of the selective OCT2 marker substrate 14C-creatinine by HEK-OCT2 cells, with a mean IC50 of 2.1 μg/mL. This is higher than vandetanib plasma concentrations (0.81 μg/mL) observed after multiple dosing at 300 mg. Inhibition of renal excretion of creatinine by vandetanib provides an explanation for increases in plasma creatinine seen in human subjects receiving vandetanib.
Specific Populations
Effects of Age and Gender
In a population pharmacokinetic evaluation in cancer patients, no relationship was apparent between oral clearance of vandetanib and patient age or gender.
Ethnicity
Based on a cross-study comparison in a limited number of patients, Japanese (N=3) and Chinese (N=7) patients had average exposures of vandetanib that were higher than Caucasian (N=7) patients receiving the same dose of CAPRELSA.
Pediatric
The pharmacokinetics of vandetanib has not been evaluated in pediatric patients.
Effect of Renal Impairment
The pharmacokinetics of vandetanib were evaluated after a single CAPRELSA dose of 800 mg in six subjects with mild (creatinine clearance = 50 to < 80 mL/min), eight subjects with moderate (creatinine clearance ≥30 to <50 mL/min), six subjects with severe (creatinine clearance < 30 mL/min) renal impairment or and ten subjects with normal (creatinine clearance > 80 mL/min) renal function. Subjects with mild renal impairment had a comparable mean AUC of vandetanib to that with normal renal function. In subjects with moderate or severe renal impairment, the average AUC of vandetanib increased by 39% and 41%, respectively, compared to patients with normal renal function [see Dosage and Administration (2.1), Warnings and Precautions (5.12) and Use in Specific Populations (8.6)].
Drug Interactions
Effect of Other Drugs on CAPRELSA
Strong CYP3A4 inducers: In a cross-over study in 12 healthy volunteers, a single oral 300 mg dose of CAPRELSA was administered alone on day 1 and on day 10 in combination with daily doses of 600 mg of rifampicin (a strong CYP3A4 inducer) given on days 1-31. The coadministration of rifampicin with CAPRELSA decreased the geometric mean AUC0-504h of vandetanib by 40% (90% confidence interval (CI): 56%, 63%) compared to vandetanib alone. No clinically meaningful change in the mean Cmax of vandetanib was observed. The geometric mean AUC0-504h and Cmax of N-desmethylvandetanib increased by 266% and 414%, respectively, in the presence of rifampicin compared with vandetanib alone [seeDrug Interactions (7.1)].
Strong CYP3A4 inhibitors: In a cross-over study in 14 healthy volunteers, a single oral 300 mg dose of CAPRELSA was administered alone and on day 4 in combination with daily doses of 200 mg of itraconazole (a strong CYP3A4 inhibitor) given on days 1-24. No change was observed in the geometric mean AUC0-504h or Cmax of vandetanib when itraconazole was coadministered with CAPRELSA.
Gastric pH elevating agents: In a cross-over study of 14 healthy volunteers, a single oral 300 mg dose of CAPRELSA was administered alone and in combination with five daily doses of 40 mg omeprazole (a proton pump inhibitor). No clinically meaningful change was observed in the geometric mean AUC0-504h and Cmax of vandetanib when omeprazole was coadministered with CAPRELSA.
In a cross-over study of 16 healthy volunteers, a single 300 mg oral dose of CAPRELSA was administered alone and after two oral doses of 150 mg of ranitidine (a H2 receptor antagonist) administered about 12 hours apart. No change was observed in the geometric mean AUC0-504h and Cmax of vandetanib when ranitidine was coadministered with CAPRELSA.
Effect of CAPRELSA on Other Drugs
Sensitive CYP3A4 substrates: In a cross-over study of 16 healthy volunteers, a single oral 7.5 mg dose of midazolam (as 2 mg/mL oral syrup), a sensitive CYP3A4 substrate, was administered alone and 8 days after receiving a single 800 mg oral dose of CAPRELSA. No change was observed in the geometric mean Cmax and AUCinf of midazolam when CAPRELSA was coadministered with midazolam.
Substrates of OCT2 transporter: In a cross-over study of 13 healthy volunteers, a single 1000 mg oral dose of metformin, a substrate of OCT2, was administered alone and 3 hours after receiving a single 800 mg oral dose of CAPRELSA. The coadministration of CAPRELSA with metformin increased the geometric mean AUCinf of metformin by 74% (90% CI: 58%, 92%) and geometric mean Cmax of metformin by 50% (90% CI: 34%, 67%) compared to metformin alone [seeDrug Interactions (7.2)].
Substrates of P-glycoprotein transporter: In a cross-over study of 14 healthy volunteers, a single oral 0.25 mg dose of digoxin, a substrate of P-glycoprotein, was administered alone and in combination with a single 300 mg oral dose of CAPRELSA. The coadministration of CAPRELSA increased the geometric mean Cmax digoxin by 29% (90% CI: 10%, 52%) and the geometric mean of AUC0-t of digoxin by 23% (90% CI: 12%, 34%) compared to digoxin alone [seeDrug Interactions (7.3)].
13. NONCLINICAL TOXICOLOGY
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies have not been conducted with vandetanib. Vandetanib was not mutagenic in vitro in the bacterial reverse mutation (Ames) assay and was not clastogenic in either the in vitro cytogenetic assay using human lymphocytes or in the in vivo rat micronucleus assay.
Based on nonclinical findings, male and female fertility may be impaired by treatment with CAPRELSA. In a fertility study of male rats, vandetanib had no effect on copulation or fertility rate when untreated females were mated with males administered 1, 5, or 20 mg/kg/day of vandetanib (approximately 0.03, 0.22, or 0.40 times, respectively, the AUC in patients with cancer at the recommended human dose of 300 mg/day); however, in the same study there was a slight decrease in the number of live embryos in females mated with males treated at the 20 mg/kg/day dose level and an increase in preimplantation loss in females mated with males administered vandetanib at doses of ≥5 mg/kg/day. In a female fertility study, there was a trend towards increased estrus cycle irregularity, a slight reduction in pregnancy incidence and an increase in implantation loss. In a one month repeat-dose toxicity study in rats, there was a decrease in the number of corpora lutea in the ovaries of rats administered 75 mg/kg/day vandetanib (approximately 1.8 times the exposure measured by AUC in patients with cancer at the recommended human dose).
13.2 Animal Toxicology and/or Pharmacology
In an animal model of wound-healing, mice dosed with vandetanib had reduced skin-breaking strength compared with controls. This suggests that CAPRELSA slows but does not prevent wound healing. The appropriate interval between discontinuation of CAPRELSA and subsequent elective surgery required to avoid the risks of impaired wound healing has not been determined.
Nodular masses were observed in a 6-month toxicology study in rats during treatment with ≥5 mg/kg/day vandetanib (approximately 0.22 or 0.40 times, respectively, the AUC in patients with cancer at the recommended human dose of 300 mg/day). Masses were palpable during clinical assessments as early as week 13, were observed in multiple organs, and were associated with hemorrhagic or inflammatory findings.
14. CLINICAL STUDIES
A double-blind, placebo-controlled study randomized patients with unresectable locally advanced or metastatic medullary thyroid cancer to CAPRELSA 300 mg (n=231) versus placebo (n=100).
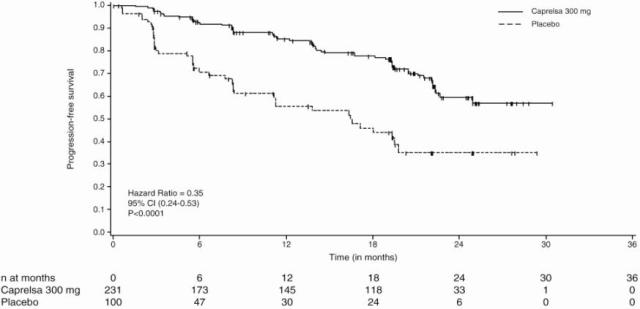
The primary objective was demonstration of improvement in progression-free survival (PFS) with CAPRELSA compared to placebo. Other endpoints included evaluation of overall survival and overall objective response rate (ORR). Centralized, independent blinded review of the imaging data was used in the assessment of PFS and ORR. Upon objective disease progression based on the investigator’s assessment, patients were discontinued from blinded study treatment and given the option to receive open-label CAPRELSA. Nineteen percent (44/231) of the patients initially randomized to CAPRELSA opted to receive open-label CAPRELSA after disease progression, and 58% (58/100) of the patients initially randomized to placebo opted to receive open-label CAPRELSA after disease progression.
The result of the PFS analysis, based on the central review RECIST assessment, showed a statistically significant improvement in PFS for patients randomized to CAPRELSA (Hazard Ratio (HR) = 0.35; 95% Confidence Interval (CI) = 0.24-0.53; p<0.0001). Analyses in the subgroups of patients who were symptomatic or had progressed within 6 months prior to their enrollment showed similar PFS results (HR = 0.31 95% CI: 0.19, 0.53 for symptomatic patients; HR = 0.41 95% CI: 0.25, 0.66 for patients who had progressed within 6 months prior to enrollment).
At the time of the primary analysis of PFS, 15% of the patients had died and there was no significant difference in overall survival between the two treatment groups. The overall objective response rate (ORR) for patients randomized to CAPRELSA was 44% compared to 1% for patients randomized to placebo. All objective responses were partial responses.
| PROGRESSION-FREE SURVIVAL | N* | Median PFS (95% CI) | HR† | 95% CI | p-value‡ |
|---|---|---|---|---|---|
|
CAPRELSA 300 mg |
59/231 (26%) |
Not reached (22.6 months, NE§) | |||
|
0.35 |
0.24, 0.53 |
<0.0001 |
|||
|
Placebo |
41/100 (41%) |
16.4 Months (8.3, 19.7) | |||
16. HOW SUPPLIED/STORAGE AND HANDLING
100 mg Tablets Available in bottles containing 30 tablets (NDC 0310–7820–30).
300 mg Tablets Available in bottles containing 30 tablets (NDC 0310–7840–30).
16.1 Storage and Handling
CAPRELSA tablets should be stored at 25°C (77°F); excursions permitted to 15°C – 30°C (59°F – 86°F) [See USP controlled room temperature].
Procedures for proper handling and disposal of anticancer drugs should be considered. A guideline on this subject has been published.1 Do not crush CAPRELSA tablets.
17. PATIENT COUNSELING INFORMATION
SEE FDA-APPROVED PATIENT LABELING (MEDICATION GUIDE)
- •
- QT Prolongation and Torsades de Pointes: Advise patients to contact their healthcare provider in the event of syncope, pre-syncopal symptoms, and cardiac palpitations. Advise patients that their healthcare provider will monitor their electrolytes and ECGs during treatment [seeWarnings and Precautions (5.1)].
- •
- Severe skin reactions and Stevens-Johnson Syndrome: Advise patients to contact their healthcare provider in the event of skin reactions or rash [seeWarnings and Precautions (5.2)].
- •
- Interstitial Lung Disease (ILD): Advise patients to contact their health care provider in the event of sudden onset or worsening of breathlessness, persistent cough or fever [seeWarnings and Precautions (5.3)].
- •
- Diarrhea: Advise patients to contact their healthcare provider in the event of diarrhea [seeWarnings and Precautions (5.7)].
- •
- Reversible Posterior Leukoencephalopathy Syndrome (RPLS): Advise patients to contact their healthcare provider in the event of seizures, headaches, visual disturbances, confusion or difficulty thinking [seeWarnings and Precautions (5.10)].
- •
- Fetal Toxicity: Because CAPRELSA can cause fetal harm, advise patients of reproductive potential to use effective contraception during therapy and for at least four months following their last dose of CAPRELSA, and to immediately contact their health care provider if pregnancy is suspected or confirmed [seeUse in Specific Populations (8.1),(8.8)].
- •
- Nursing Infants: Because of the potential for serious adverse reactions in nursing infants from CAPRELSA, advise breast feeding mothers to discontinue nursing while receiving therapy [seeUse in Specific Populations (8.3)].
- •
- Photosensitivity: Advise patients to use appropriate sun protection due to the increased susceptibility to sunburn while taking CAPRELSA and for at least 4 months after drug discontinuation [seeWarnings and Precautions (5.2)].
- •
- Administration: Advise patients that CAPRELSA can be taken with or without food and not to crush CAPRELSA tablets [seeClinical Pharmacology (12.3)].
MEDICATION GUIDE
CAPRELSA® (kap-rel-sah)
(vandetanib)
Tablets
Read this Medication Guide before you start taking CAPRELSA and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking to your healthcare provider about your medical condition or treatment.
What is the most important information I should know about CAPRELSA?
CAPRELSA can cause a change in the electrical activity of your heart called QT prolongation, which can cause irregular heartbeats and that may lead to death. You should not take CAPRELSA if you have had a condition called long QT syndrome since birth.
Your healthcare provider should perform tests to check the levels of your blood potassium, calcium, magnesium, and thyroid-stimulating hormone (TSH) as well as the electrical activity of your heart with a test called an electrocardiogram (ECG). You should have these tests:
- •
- Before starting CAPRELSA
- •
- Regularly during CAPRELSA treatment:
- o
- 2 to 4 weeks after starting CAPRELSA
- o
- 8 to 12 weeks after starting CAPRELSA
- o
- Every 3 months thereafter
- o
- If your healthcare provider changes your dose of CAPRELSA
- o
- If you start taking medicine that causes QT prolongation
- o
- As instructed by your healthcare provider
Your healthcare provider may stop your CAPRELSA treatment for a while and restart you at a lower dose if you have QT prolongation.
Call your healthcare provider right away if you feel faint, light-headed, or feel your heart beating irregularly while taking CAPRELSA. These may be symptoms related to QT prolongation.
What is CAPRELSA?
CAPRELSA is a prescription medicine used to treat medullary thyroid cancer that cannot be removed by surgery or that has spread to other parts of the body. It takes a long time to get rid of CAPRELSA from your body and you may be at risk for side effects related to CAPRELSA after you have stopped your treatment.
It is not known if CAPRELSA is safe and effective in children.
Who should not take CAPRELSA?
Do not take CAPRELSA if you have had QT prolongation.
What should I tell my healthcare provider before taking CAPRELSA?
Before you take CAPRELSA, tell your healthcare provider if you:
- •
- have any heart problems, including a condition called congenital long QT syndrome
- •
- have an irregular heartbeat
- •
- take or have stopped taking a medicine that causes QT prolongation
- •
- have low blood levels of potassium, calcium, or magnesium
- •
- have high blood levels of thyroid-stimulating hormone
- •
- have high blood pressure
- •
- have skin problems
- •
- have a history of breathing problems
- •
- have a recent history of coughing up blood or bleeding
- •
- have diarrhea
- •
- have liver problems
- •
- have kidney problems
- •
- have seizures or are being treated for seizures
- •
- are pregnant or plan to become pregnant. CAPRELSA can cause harm to your unborn baby. Talk to your healthcare provider if you are pregnant or plan to become pregnant.
- o
- If you are able to become pregnant, you should use effective birth control during your treatment with CAPRELSA and for at least 4 months after your last dose of CAPRELSA.
- o
- Talk to your healthcare provider about birth control methods to prevent pregnancy while you are taking CAPRELSA.
- •
- are breastfeeding or plan to breastfeed. It is not known if CAPRELSA passes into your breast milk. You and your healthcare provider should decide if you will take CAPRELSA or breastfeed. You should not do both.
Tell your healthcare provider about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements. CAPRELSA and other medicines may affect each other causing side effects.
Especially tell your healthcare provider if you take:
- •
- St. John’s Wort. You should not take St. John’s Wort while taking CAPRELSA
- •
- certain medicines that can affect how your liver breaks down medicine
- •
- a medicine for your heart
Ask your healthcare provider if you are not sure if your medicine is one listed above.
Do not take other medicines while taking CAPRELSA until you have talked with your healthcare provider or pharmacist.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take CAPRELSA?
- •
- Take CAPRELSA exactly as your healthcare provider tells you to take it. Do not change your dose or stop taking CAPRELSA unless your healthcare provider tells you to.
- •
- CAPRELSA may be taken with or without food.
- •
- Swallow CAPRELSA tablets whole with water.
- •
- Do not crush or chew CAPRELSA tablets. If CAPRELSA tablets are accidentally crushed, contact with skin should be avoided. If contact occurs, wash affected areas well with water.
- •
- If you cannot swallow CAPRELSA tablets whole:
- o
- place your dose of CAPRELSA in a glass that contains 2 ounces of noncarbonated water (no other liquids should be used).
- o
- stir the CAPRELSA tablet(s) and water mixture for about 10 minutes or until the tablet(s) are in very small pieces (the tablets will not completely dissolve).
- o
- swallow CAPRELSA and water mixture right away.
- o
- if any CAPRELSA and water mixture remains in the glass, mix with an additional 4 ounces of noncarbonated water and swallow the mixture to make sure that you take your full dose of CAPRELSA.
- •
- If you miss a dose and your next dose is in:
- o
- less than 12 hours, take your next dose at the normal time. Do not make up for the missed dose.
- o
- 12 hours or more, take the missed dose as soon as you remember. Take the next dose at the normal time.
Call your healthcare provider right away if you take too much CAPRELSA.
- •
- During treatment with CAPRELSA, your healthcare provider should check your blood and heart for side effects. See “What is the most important information I should know about CAPRELSA?”
- •
- Your healthcare provider should check your blood pressure regularly during your treatment with CAPRELSA.
What should I avoid while taking CAPRELSA?
- •
- Limit exposure to the sun. CAPRELSA can make your skin sensitive to the sun. While taking CAPRELSA and for 4 months after stopping your CAPRELSA treatment, use sun block and wear clothes that cover your skin, including your head, arms and legs when you go outdoors.
- •
- Use caution before driving or using machinery. Keep in mind CAPRELSA may make you feel tired, weak, or cause blurred vision.
What are the possible side effects of CAPRELSA?
CAPRELSA may cause serious side effects, including:
- •
- See “What is the most important information I should know about CAPRELSA?”
- •
-
Serious skin reactions. CAPRELSA can cause a serious skin reaction, called Stevens-Johnson syndrome or other serious skin reactions that may affect any part of your body. These serious skin reactions may be life threatening and you may need to be treated in a hospital. Call your healthcare provider right away if you experience any of these symptoms.
- o
- Skin rash or acne
- o
- Dry skin
- o
- Itching
- o
- Blisters on your skin
- o
- Blisters or sores in your mouth
- o
- Peeling of your skin
- o
- Fever
- o
- Muscle or joint aches
- o
- Redness or swelling of your face, hands, or soles of your feet
- •
- Breathing problems (interstitial lung disease). CAPRELSA may cause a breathing problem called interstitial lung disease that can lead to death. Tell your healthcare provider right away if you experience sudden or worsening shortness of breath or cough.
- •
-
Stroke. Strokes have been reported in some people who have taken CAPRELSA and in some cases have caused death. Stop taking CAPRELSA and call your healthcare provider right away if you have symptoms of a stroke which may include:
- o
- numbness or weakness of the face, arm or leg, especially on one side of the body
- o
- sudden confusion, trouble speaking or understanding
- o
- sudden trouble seeing in one or both eyes
- o
- sudden trouble walking, dizziness, loss of balance or coordination
- o
- sudden, severe headache
- •
- Bleeding. Bleeding can happen during your treatment with CAPRELSA. Tell your healthcare provider right away if you have severe bleeding while you are taking CAPRELSA.
- •
- Heartfailure. CAPRELSA can cause heart failure that can lead to death. You may have to stop taking CAPRELSA if you have heart failure. Heart failure may not be reversible after stopping CAPRELSA. Your healthcare provider should monitor you for signs and symptoms of heart failure.
- •
- Diarrhea. Diarrhea is often a symptom of medullary thyroid cancer. CAPRELSA can also cause diarrhea or make diarrhea worse. Your healthcare provider should check your blood levels to monitor your electrolytes more frequently if you have diarrhea.
- •
- Thyroidhormones. You can have changes in your thyroid hormone when taking CAPRELSA. Your healthcare provider should monitor your thyroid hormone levels while taking CAPRELSA.
- •
- High blood pressure (hypertension). If you develop high blood pressure or your high blood pressure gets worse, your healthcare provider may lower your dose of CAPRELSA or tell you to stop taking CAPRELSA until your blood pressure is under control. Your healthcare provider may prescribe another medicine to control your high blood pressure.
- •
-
Reversible Posterior Leukoencephalopathy Syndrome (RPLS). A condition called reversible posterior leukoencephalopathy syndrome can happen while taking CAPRELSA. Call your healthcare provider right away if you have:
- o
- headaches
- o
- seizures
- o
- confusion
- o
- changes in vision
- o
- problems thinking
The most common side effects of CAPRELSA include:
-
- o
- diarrhea
- o
- rash
- o
- acne
- o
- nausea
- o
- high blood pressure
- o
- headache
- o
- feeling tired
- o
- loss of appetite
- o
- upper respiratory tract infections
- o
- stomach (abdominal) pain
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of CAPRELSA. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store CAPRELSA?
- •
- Store CAPRELSA tablets at 59°F to 86°F (15°C to 30°C).
- •
- Safely throw away medicine that is out of date or that you no longer need. Ask your pharmacist how to safely throw away CAPRELSA tablets.
Keep CAPRELSA and all medicines out of the reach of children.
General information about CAPRELSA.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use CAPRELSA for a condition for which it was not prescribed. Do not give CAPRELSA to other people, even if they have the same symptoms you have. It may harm them.
This Medication Guide summarizes important information about CAPRELSA. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about CAPRELSA that is written for health professionals.
For more information, go to www.caprelsa.com or call 1-800-236-9933.
What are the ingredients in CAPRELSA?
Active ingredient: vandetanib
Inactive ingredients:
- •
- Tablet core: calcium hydrogen phosphate dihydrate, microcrystalline cellulose, crospovidone, povidone, and magnesium stearate
- •
- Tablet film-coat: hypromellose 2910, macrogol 300, and titanium dioxide E171
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Distributed by:
AstraZeneca Pharmaceuticals LP
Wilmington, DE 19850
Issued: 03/2016
CAPRELSA is a registered trademark of the AstraZeneca group of companies
©AstraZeneca 2013. All Rights Reserved.
| CAPRELSA
vandetanib tablet |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| CAPRELSA
vandetanib tablet |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - AstraZeneca Pharmaceuticals LP (054743190) |
| Registrant - AstraZeneca PLC (230790719) |