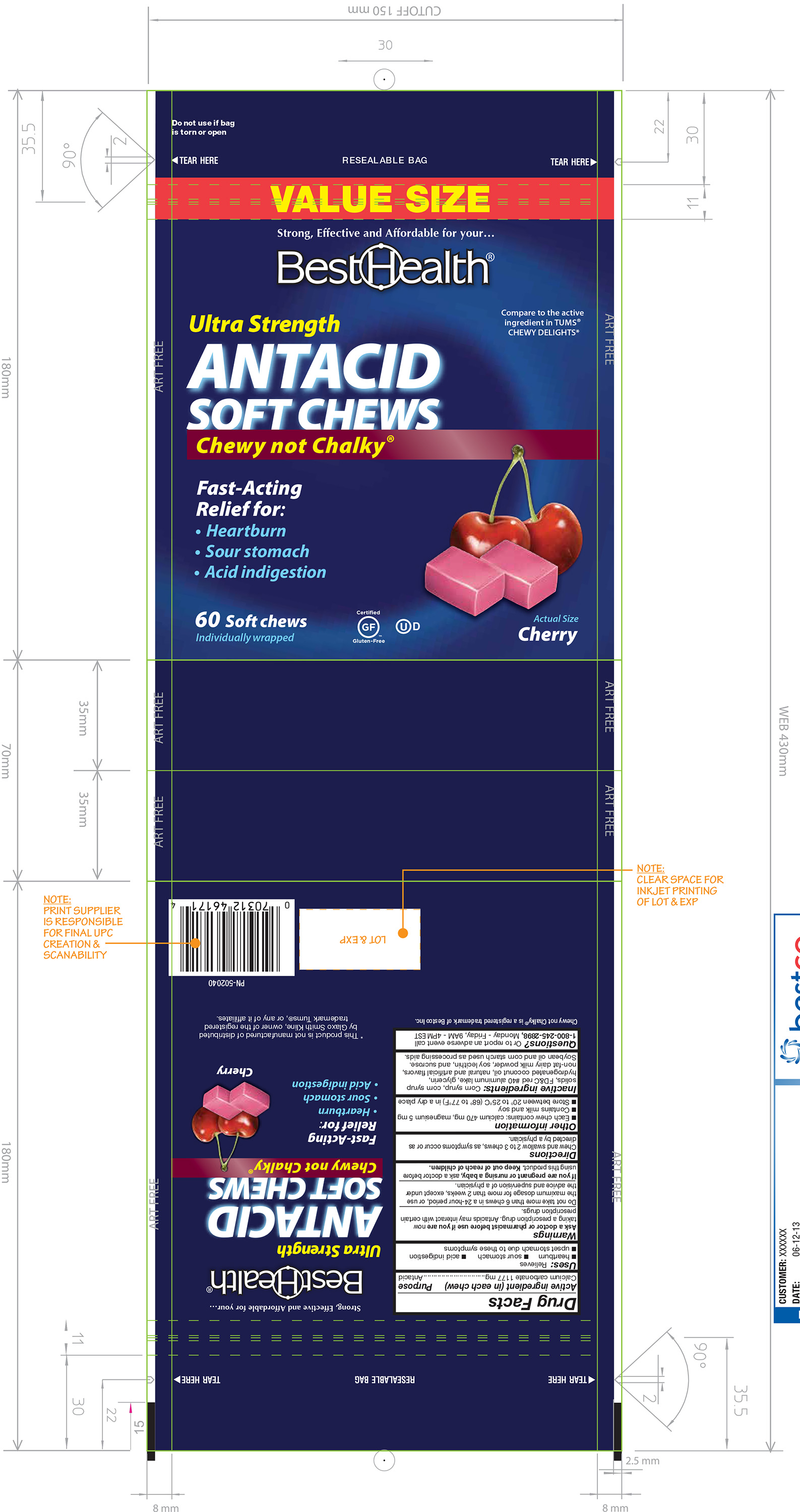
CHERRY ANTACID SOFT CHEWS- calcium carbonate tablet, chewable
BestCo Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Calcium Carbonate 1177 mg
Warnings
Ask a doctor or pharmacist before use if you are now taking a prescription drug. Antacids may interact with certain prescription drugs. Do not take more than 6 chews in a 24-hour period, or use the maximum dosage for more than 2 weeks, except under the advice and supervision of a physician.
Other information
Each chew contains: calcium 470 mg, magnesium 5 mg
Contains milk and soy
Store between 20º to 25ºC (68º to 77ºF) in a dry place
| CHERRY ANTACID SOFT CHEWS
calcium carbonate tablet, chewable |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
 |
||||||||||||||||||
|
||||||||||||||||||
| Labeler - BestCo Inc. (002149136) |
| Registrant - BestCo Inc. (002149136) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BestCo Inc. | 002149136 | manufacture(52642-022) | |
Revised: 7/2022
Document Id: e33d98c4-c5cc-33d2-e053-2995a90a147c
Set id: 4c6c7b25-b8b5-483a-e054-00144ff8d46c
Version: 2
Effective Time: 20220707
BestCo Inc.