MY SHIELD SANITIZING BATH- benzalkonium chloride swab
ESC Brands LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
My-Shield Sanitizing Bath Wipe
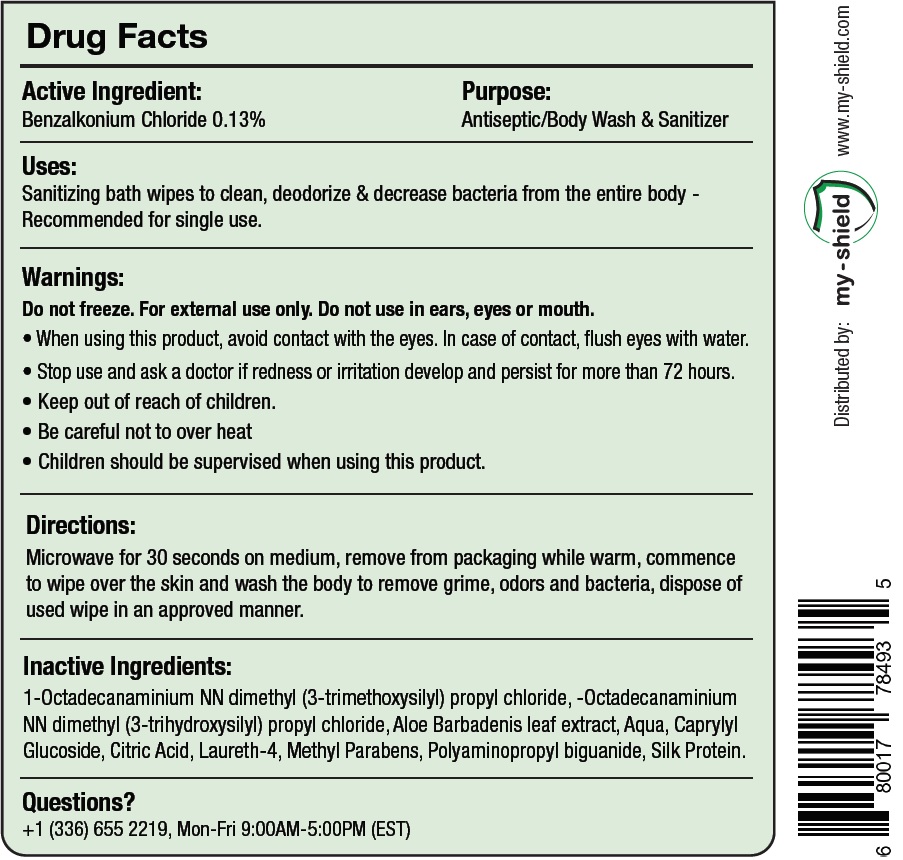
Uses:
Sanitizing bath wipes to clean, deodorize & decrease bacteria from the entire body - Recommended for single use.
Directions:
Microwave for 30 seconds on medium, remove from packaging while warm, commence to wipe over the skin and wash the body to remove grime, odors and bacteria, dispose of used wipe in an approved manner.
| MY SHIELD SANITIZING BATH
benzalkonium chloride swab |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - ESC Brands LLC (202621850) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Filltech USA, LLC | 926433855 | manufacture(71884-004) | |
Revised: 10/2021
Document Id: cdc4e8e4-b6d7-395a-e053-2995a90a7fbd
Set id: 4c20af86-8642-434b-8407-ccd61632cfe4
Version: 6
Effective Time: 20211007
ESC Brands LLC