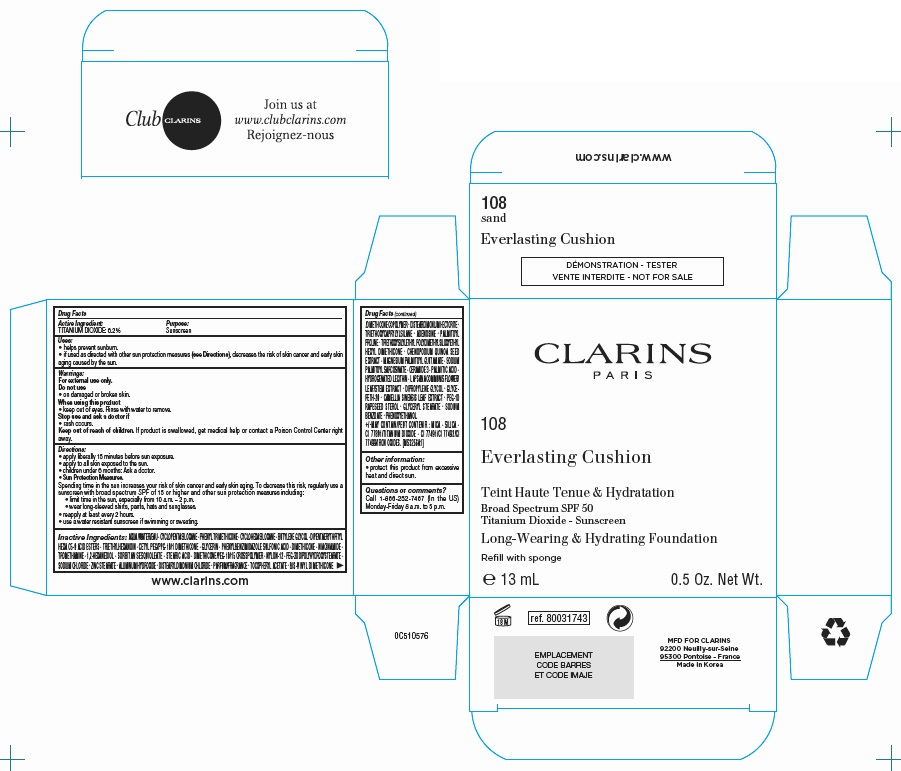
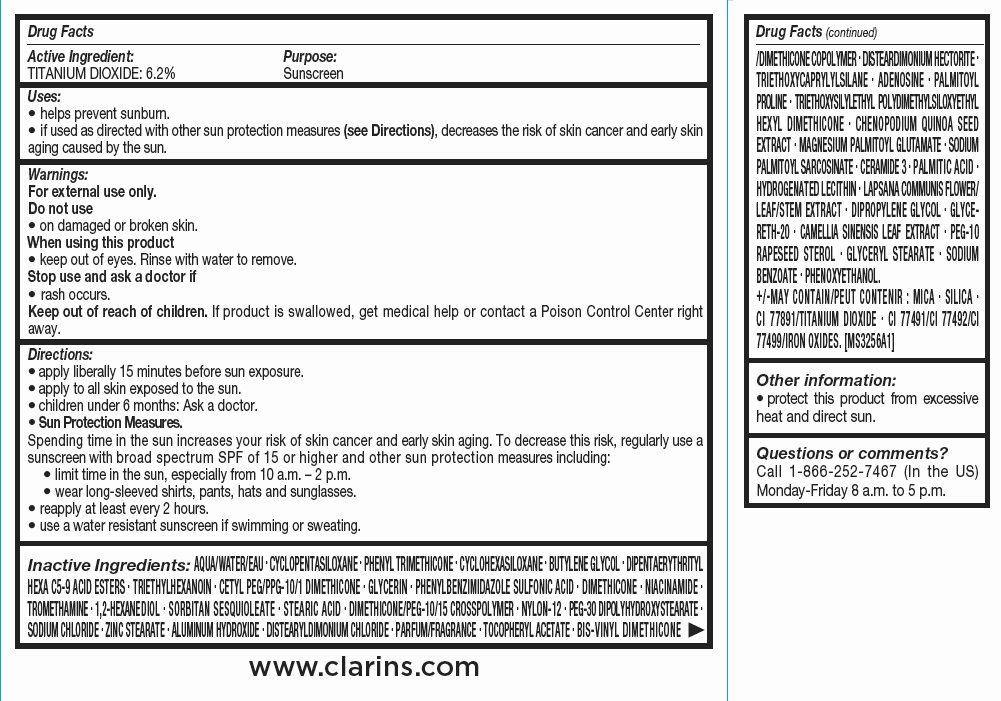
CLARINS EVERLASTING CUSHION LONG-WEARING AND HYDRATING FOUNDATION BROAD SPECTRUM SPF 50 TINT 108 TESTER REFILL WITH SPONGE- titanium dioxide emulsion
Laboratoires Clarins
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Clarins Everlasting Cushion Long-Wearing and Hydrating Foundation Broad Spectrum SPF 50 Tint 108 - Tester refill with sponge
| CLARINS EVERLASTING CUSHION LONG-WEARING AND HYDRATING FOUNDATION BROAD SPECTRUM SPF 50 TINT 108
TESTER REFILL WITH SPONGE
titanium dioxide emulsion |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Laboratoires Clarins (266317555) |
Revised: 11/2021
Document Id: d0557af2-0f28-63c7-e053-2a95a90a8055
Set id: 4b70fbb7-4a2f-4b88-a668-223b9168b8b8
Version: 4
Effective Time: 20211109
Laboratoires Clarins