SINUS- oyster shell calcium carbonate, crude, goldenseal, potassium dichromate, and pulsatilla vulgaris tablet
Hyland's Inc.
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
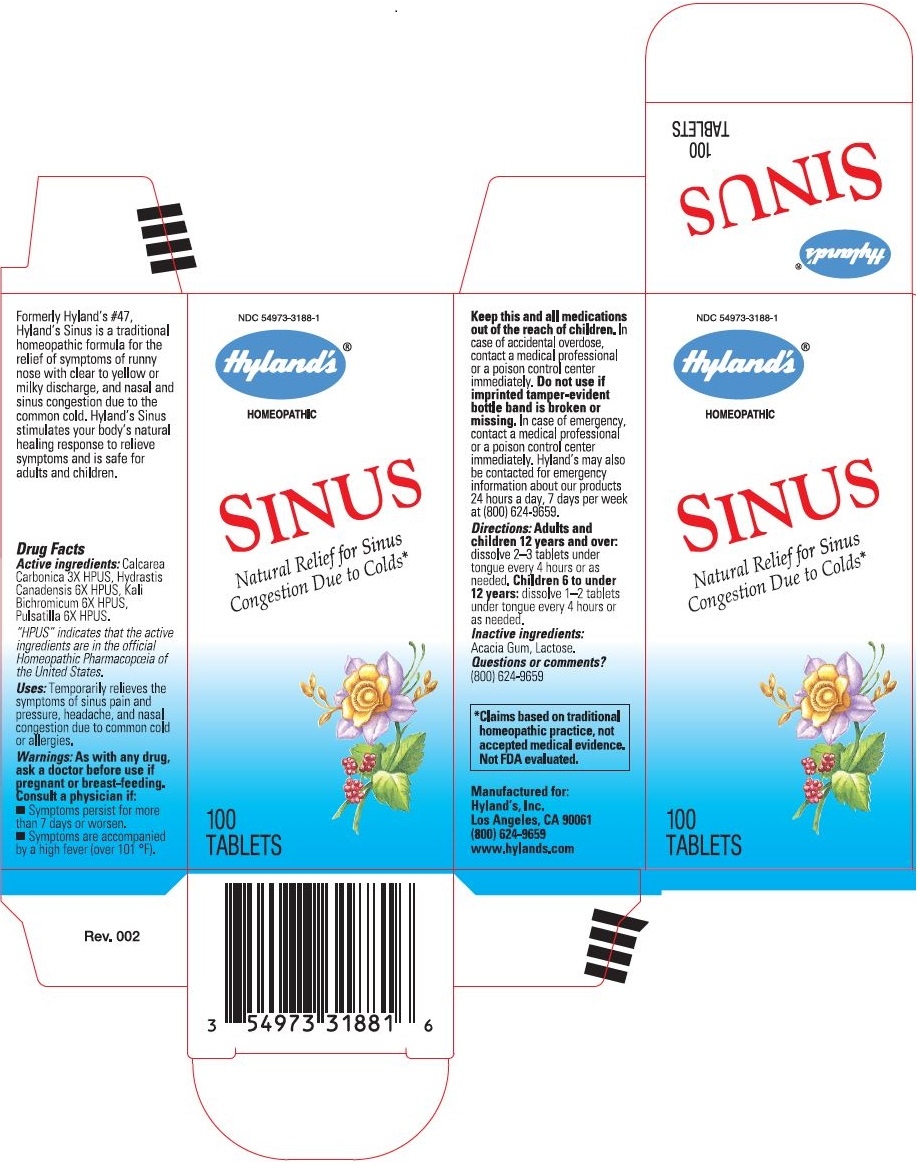
Formerly Hyland's #47, Hyland's Sinus is a traditional homeopathic formula for the relief of symptoms of runny nose with clear to yellow or milky discharge, and nasal and sinus congestion due to the common cold. Hyland's Sinus stimulates your body's natural healing response to relieve symptoms and is safe for adults and children.
Drug Facts
Active ingredients
Calcarea Carbonica 3X HPUS, Hydrastis Canadensis 6X HPUS, Kali Bichromicum 6X HPUS, Pulsatilla 6X HPUS
“HPUS” indicates that the active ingredients are in the
official Homeopathic Pharmacopoeia of the United States.
Uses
Temporarily relieves the symptoms of sinus pain and pressure, headache, and nasal congestion due to common cold or allergies.
Warnings
As with any drug, ask a doctor before use if pregnant or breast-feeding.
Consult a physician if
- Symptoms persist for more than 7 days or worsen.
- Symptoms are accompanied by a high fever (over 101 °F).
Keep this and all medications out of the reach of children.In case of accidental overdose, contact a medical professional or a poison control center immediately.
Do not use if imprinted tamper-evident bottle band is broken or missing.
In case of emergency, contact a medical professional or a poison control center immediately. Hyland’s may also be contacted for emergency information about our products 24 hours a day, 7 days per week at (800) 624-9659.
Directions
Adults and children 12 years and over
dissolve 2-3 tablets under tongue every 4 hours or as needed.
Children 6 to under 12 years
dissolve 1–2 tablets under tongue every 4 hours or as needed.
Questions or comments?
(800) 624-9659
Inactive ingredients
Acacia Gum, Lactose.
Temporarily relieves the symptoms of sinus pain and pressure, headache, and nasal congestion due to common cold or allergies.
PRINCIPAL DISPLAY PANEL - 100 Tablet Bottle Carton
NDC 54973-3188-1
Hyland's
®
HOMEOPATHIC
SINUS
Natural Relief for Sinus
Congestion Due to Colds
100
TABLETS
Hyland's Inc.
