Label: SCRUB-STAT- chlorhexidine gluconate solution
-
NDC Code(s):
47593-467-21,
47593-467-36,
47593-467-41,
47593-467-44, view more47593-467-56, 47593-467-59
- Packager: Ecolab Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated August 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
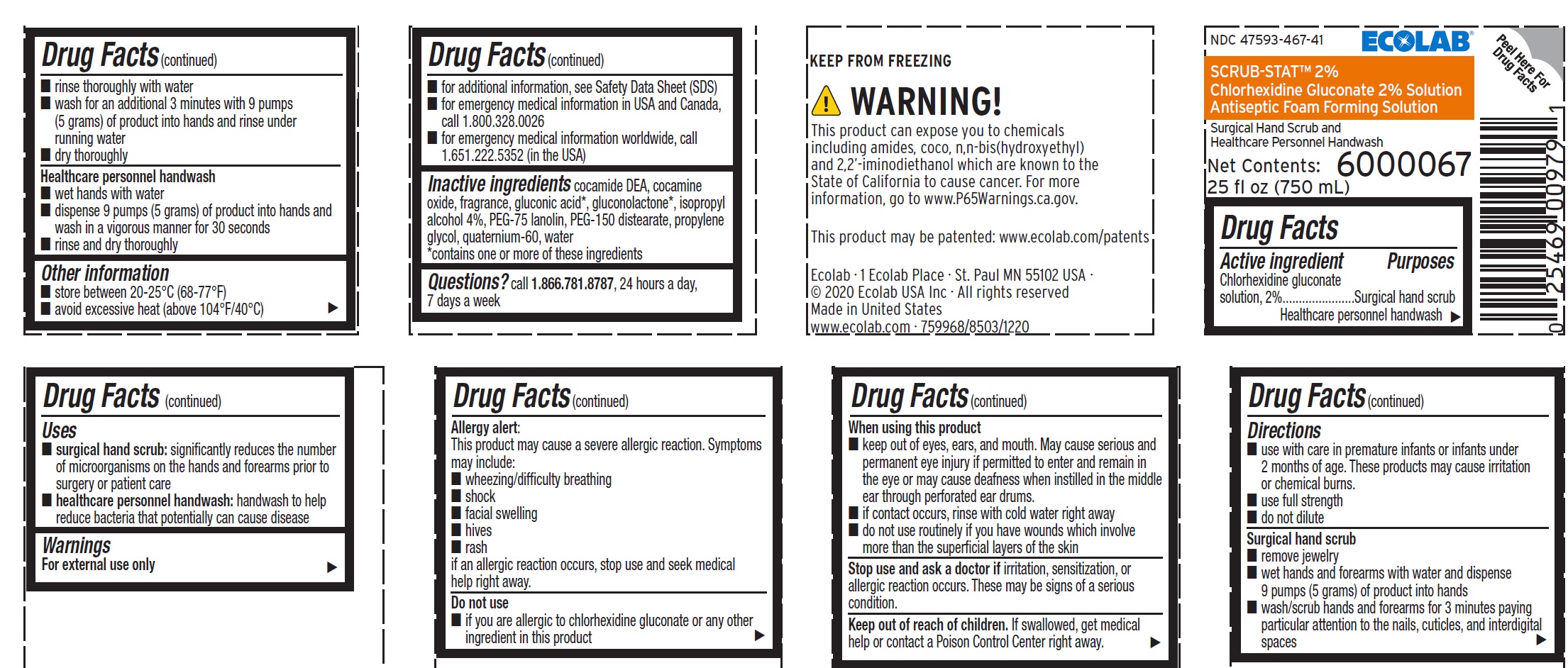
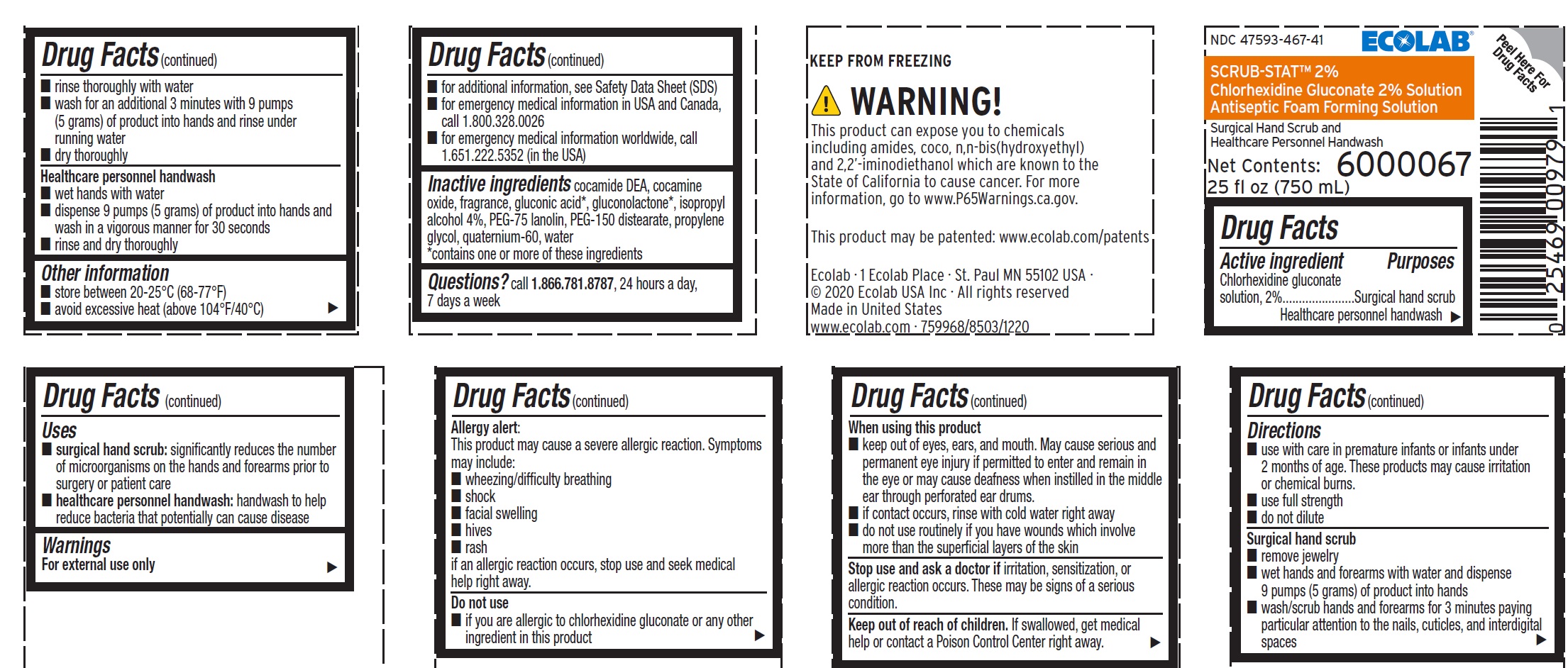
- Active ingredient
- Purposes
- Uses
-
Warnings
For external use only
Allergy Alert:
This product may cause a severe allergic reaction. Symptoms may include:
- wheezing/difficulty breathing
- shock
- facial swelling
- hives
- rash
if an allergic reaction occurs, stop use and seek medical help right away.
When using this product
- keep out of eyes, ears, and mouth. May cause serious and permanent eye injury if permitted to enter and remain in the eye or may cause deafness when instilled in the middle ear through perforated ear drums.
- if contact occurs, rinse with cold water right away
- do not use routinely if you have wounds which involve more than the superficial layers of the skin
-
Directions
- use with care in premature infants or infants under 2 months of age. These products may cause irritation or chemical burns.
- use full strength
- do not dilute
Surgical hand scrub
- remove jewelry
- wet hands and forearms with water and dispense 9 pumps (5 grams) of product into hands
- wash/scrub hands and forearms for 3 minutes paying particular attention to the nails, cuticles, and interdigital spaces
- rinse thoroughly with water
- wash for an additional 3 minutes with 9 pumps (5 grams) of product into hands and rinse under running water
- dry thoroughly
Healthcare personnel handwash
- wet hands with water
- dispense 9 pumps (5 grams) of product into hands and wash in a vigorous manner for 30 seconds
- rinse and dry thoroughly
- Other information
- INACTIVE INGREDIENT
- QUESTIONS
-
Representative label and principal display panel
ECOLAB®
NDC 47593-467-41
SCRUB-STAT™ 2%
Chlorhexidine Gluconate 2% Solution
Antiseptic Foam Forming Solution
Surgical Hand Scrub and
Healthcare Personnel Handwash
Net Contents:
25 fl oz (750 mL)
6000067
KEEP FROM FREEZING
Ecolab · 1 Ecolab Place · St Paul MN 55102 USA
© 2020 Ecolab USA Inc · All rights reserved
Made in United States
www.ecolab.com · 759968/8503/1220
-
INGREDIENTS AND APPEARANCE
SCRUB-STAT
chlorhexidine gluconate solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:47593-467 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE GLUCONATE 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength COCO DIETHANOLAMIDE (UNII: 92005F972D) COCAMINE OXIDE (UNII: QWA2IZI6FI) GLUCONIC ACID (UNII: R4R8J0Q44B) GLUCONOLACTONE (UNII: WQ29KQ9POT) ISOPROPYL ALCOHOL (UNII: ND2M416302) PEG-75 LANOLIN (UNII: 09179OX7TB) PEG-150 DISTEARATE (UNII: 6F36Q0I0AC) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) QUATERNIUM-33 (UNII: XPS4174QZJ) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47593-467-41 750 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/03/2011 2 NDC:47593-467-59 1250 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/03/2011 3 NDC:47593-467-56 1200 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/03/2011 4 NDC:47593-467-44 1134000 mL in 1 CONTAINER; Type 0: Not a Combination Product 12/22/2015 10/15/2024 5 NDC:47593-467-21 208000 mL in 1 DRUM; Type 0: Not a Combination Product 06/05/2013 10/15/2024 6 NDC:47593-467-36 800 mL in 1 BOTTLE; Type 0: Not a Combination Product 07/20/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA019258 03/03/2011 Labeler - Ecolab Inc. (006154611)