VANACOF AC- chlophedianol hcl, pyrilamine maleate liquid
GM Pharmaceuticals, Inc.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
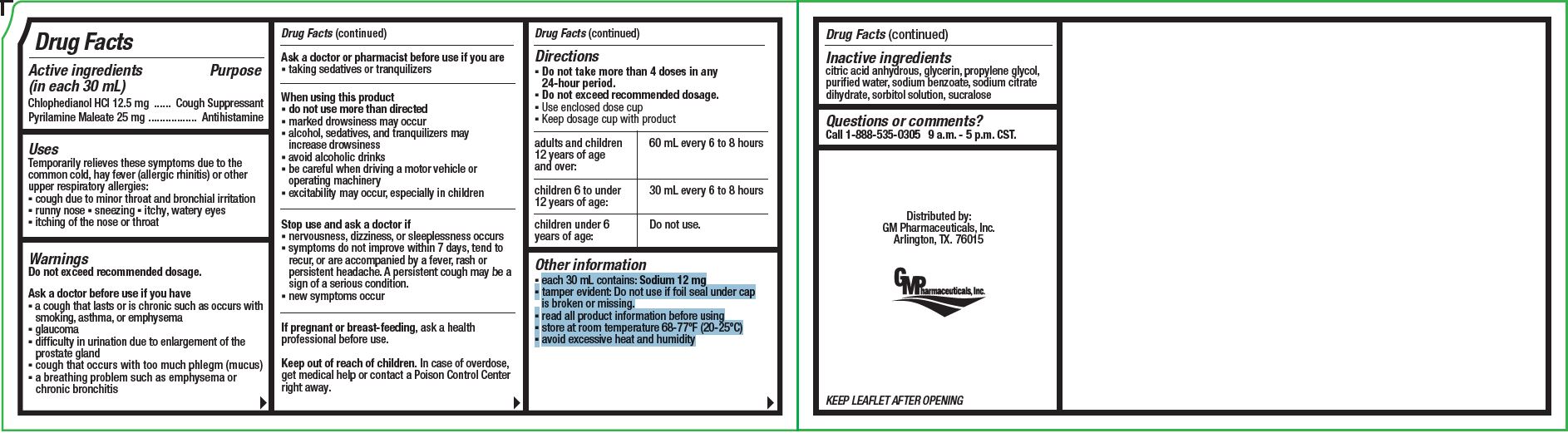
VanaCof AC
Active Ingredients
Each 30 mL contains:
Chlophedianol HCl ...........12.5
Pyrilamine Maleate ...........25
Uses
Temporarily relieves these symptoms due to the common cold, hay fever (allergic rhinitis) or other upper respiratory allergies:
- cough due to minor throat and bronchial irritation
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
Ask a doctor before use if you have
- a cough that lasts or is chronic such as occurs with smoking, asthma or emphysema
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
- cough that occurs with too much phlegm (mucus)
- a breathing problem such as emphysema or chronic bronchitis
When using this poduct
- do not use more than directed
- marked drowsiness may occur
- alcohol, sedatives, and tranquilizers may increase drowsiness
- avoid alcoholics drinks
- be careful when driving a motor vehicle or operating machinery
- excitability may occur, especially in children
Stop use and ask a doctor if:
- nervousness, dizziness or sleeplessnss occurs
- symptoms do not improve within 7 days, tend to recur, or are accompanied by a fever, rash or persistent headache. A persistent cough may be a sign of a serious condition.
- new symptoms occur
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- Do not take more than 4 doses in any 24-hour period.
- Do not exceed recommended dosage.
- Use enclosed dose cup
- Keep dosage cup with product
| adults and children 12 years of age and over: |
60 mL every 6-8 hours |
| children 6 to under 12 years of age:30 | 30 mLevery 6-8 hours |
| children under 6 years of age: | Do not use |
Other information
- each 30 mL contains: Sodium 12 mg
- tamper evident: Do not use if foil seal under cap is broken or missing.
- read all product information before using
- store at room temperature 68-77ºF (20-25ºC)
- avoid excessive heat and humidity
| VANACOF AC
chlophedianol hcl, pyrilamine maleate liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - GM Pharmaceuticals, Inc. (793000860) |
Revised: 1/2021
Document Id: b9443f27-160b-97eb-e053-2a95a90a195b
Set id: 4b42dbb6-f765-6276-e054-00144ff8d46c
Version: 3
Effective Time: 20210119
GM Pharmaceuticals, Inc.