TIS-U-SOL- sodium chloride, potassium chloride, magnesium sulfate, sodium phosphate, dibasic, heptahydrate and potassium phosphate, monobasic solution
Baxter Healthcare Company
----------
Directions for Use of Flexible Plastic Irrigation Containers
If desired, warm in overwrap to near body temperature in a water bath or oven heated to not more than 45°C.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
DIRECTIONS FOR USE
Tear overwrap down side at slit and remove solution container. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually. Check for minute leaks by squeezing bag firmly. If leaks are found, discard solution as sterility may be impaired.
Use Aseptic Technique.
1. Suspend container using hanger hole.
2. Remove plastic protector from outlet port at bottom of container.
3. Attach irrigation set. Refer to complete directions accompanying set.
Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. It is recommended the product be stored at room temperature (25°C): brief exposure up to 40°C does not adversely affect the product.
Baxter Healthcare Corporation
Deerfield, IL 60015 USA
Printed in USA
©Copyright 1980, 1984, 1989, Baxter Healthcare Corporation.
All rights reserved.
7-19-4-268
Rev. July 1997
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL
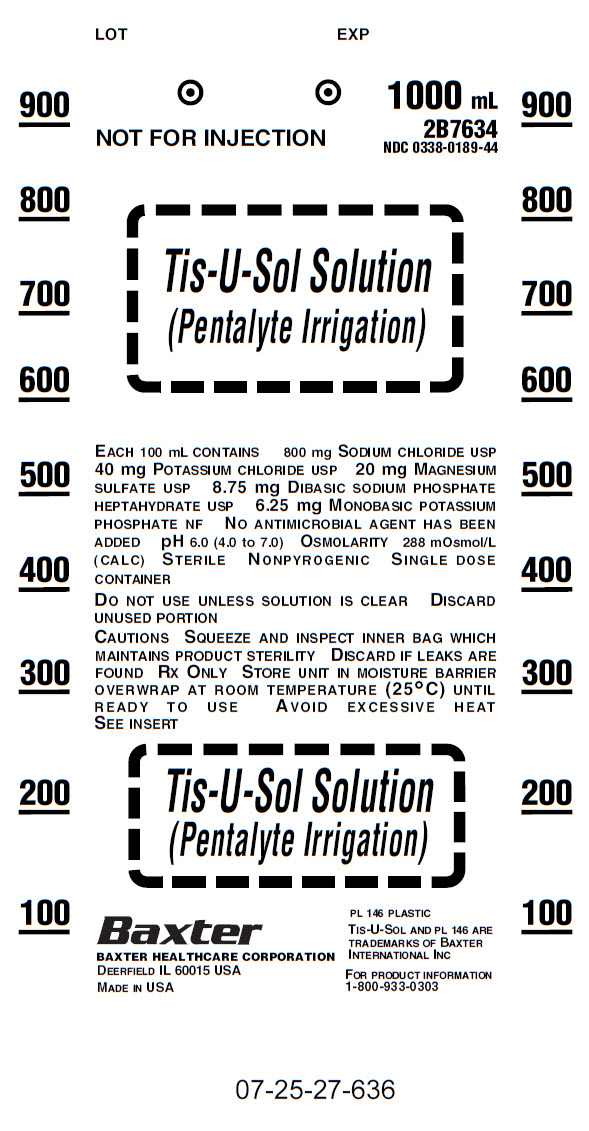
Contaner Label
900
800
700
600
500
400
300
200
100
LOT EXP
1000 mL
NOT FOR INJECTION 2B7634
NDC 0338-0189-44
Tis-U-Sol Solution
(Pentalyte Irrigation)
EACH 100 mL CONTAINER 800 mg SODIUM CHLORIDE USP
40 mg POTASSIUM CHLORIDE USP 20 mg MAGNESIUM
SULFATE USP 8.75 mg DIBASIC SODIUM PHOSPHATE
HELTAHYDRATE NF NO ANTIMICROBIAL AGENT HAS BEEN
ADDED pH 6.0 (4.0 TO 7.0) OSMOLARITY 288 mOsmol/L
(CALC) STERILE NONPYROGENIC SINGLE DOSE
CONTAINER
DO NOT USE UNLESS SOLUTION IS CLEAR DISCARD
UNUSED PORTION
CAUTIONS SQUEEZE AND INSPECT INNER BAG WHICH
MAINTAINS PRODUCT STERILITY DISCARD IF LEAKS ARE
FOUND Rx ONLY STORE UNIT IN MOISTURE BARRIER
OVERWRAP AT ROOM TEMPERATURE (25°C) UNTIL
READY TO USE AVOID EXCESSIVE HEAT
SEE INSERT
Tis-U-Sol Solution
(Pentalyte Irrigation)
Baxter Logo
BAXTER HEALTHCARE CORPORATION
DEERFIELD, IL 60015 USA
MADE IN USA
PL 146 PLASTIC
TIS-U-SOL AND PL 146 ARE
TRADEMARKS OF BAXTER
INTERNATIONAL INC
FOR PRODUCT INFORMATION
1-800-933-0303
07-25-27-636
900
800
700
600
500
400
300
200
100
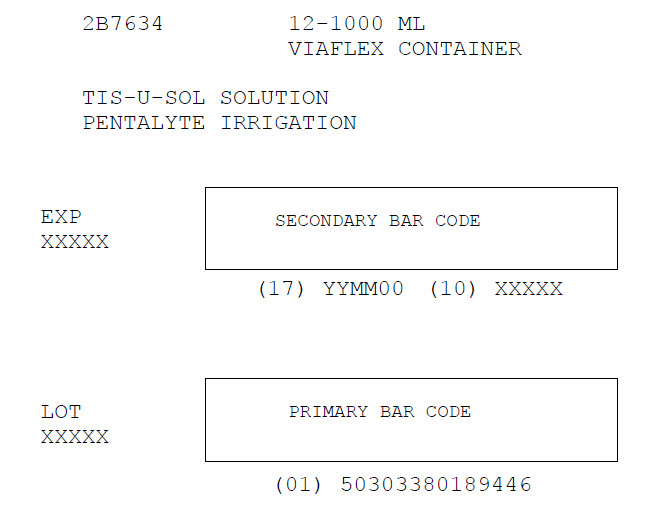
Carton Label
2B7634 12-1000 ML
VIAFLEX CONTAINER
TIS-U-SOL SOLUTION
PENTALYTE IRRIGATION
EXP
XXXXX
SECONDARY BAR CODE
(17) YYMM00 (10) XXXXX
LOT
XXXXX
PRIMARY BAR CODE
(01) 50303380189446
| TIS-U-SOL
sodium chloride, potassium chloride, magnesium sulfate, sodium phosphate and potassium phosphate solution |
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
|
|||||||||||||||||||||
| Labeler - Baxter Healthcare Company (005083209) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Baxter Healthcare Corporation | 059140764 | ANALYSIS(0338-0189), LABEL(0338-0189), MANUFACTURE(0338-0189), PACK(0338-0189), STERILIZE(0338-0189) | |