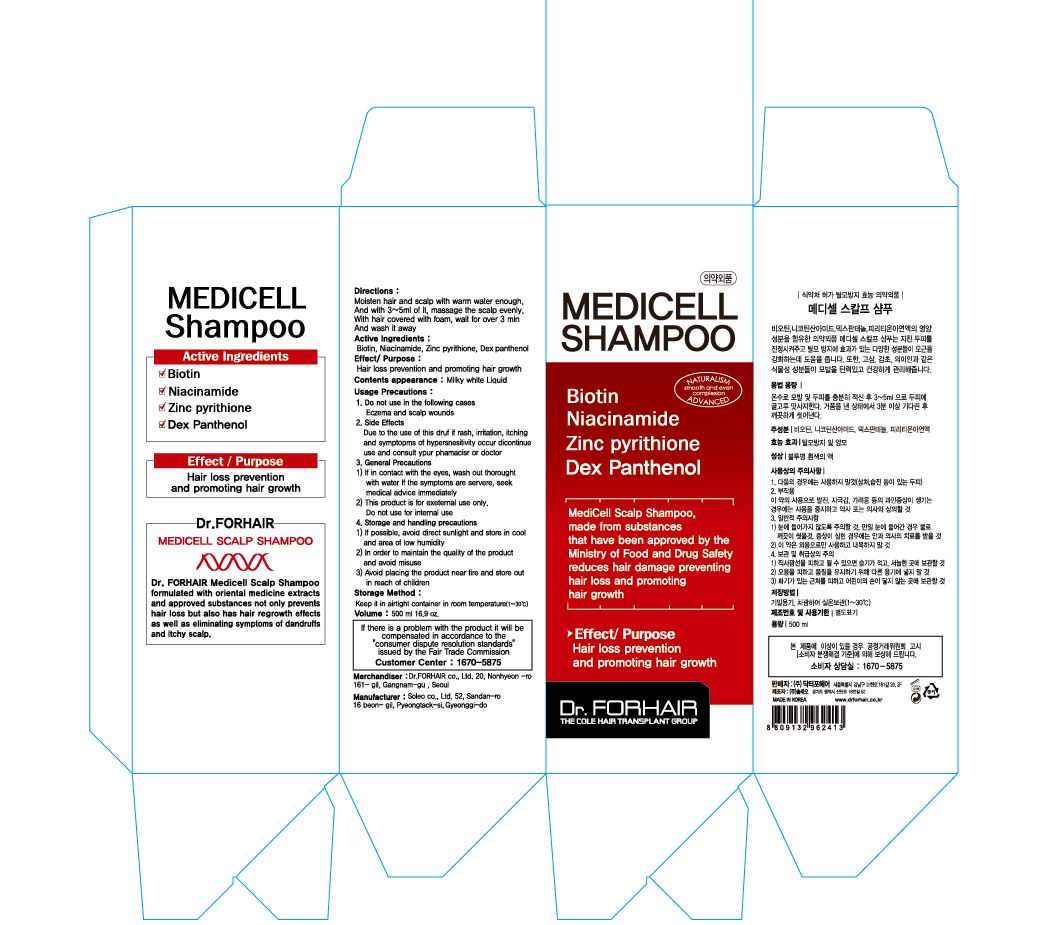
MEDICELL SCALP- glycerin shampoo
Humajor Co., Ltd.
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Water, Sodium Laureth Sulfate , Ammonium Lauryl Sulfate, Cocamidopropyl Betaine, Polyquaternium-7, Dimethicone, Zinc Pyrithione, Cocamide DEA, Panthenol, Pyrrolidinyl Diaminopyrimidine Oxide, Guar Hydroxypropyltrimonium Chloride, Niacinamide, Menthol, Cetrimonium Chloride, Zinc Chloride, Biotin, Trideceth-10, Sodium Polynaphthalenesulfonate, Butylene Glycol, Moringa Pterygosperma Seed Extract , Sophora Angustifolia Root Extract , Glycyrrhiza Glabra (Licorice) Root Extract, Coix Lacryma-Jobi Ma-yuen Seed Extract, Artemisia Princeps Leaf Extract, Carbomer,Sodium Chloride, Disodium EDTA, Citric Acid, Sodium Benzoate, Phenoxyethanol,Fragrance
hair growth and loss prevention
keep out or reach of the children
1) Sufficiently wet the scalp and hair with warm water
2) Take appropriate amount on palm and apply to wet hair and scalp. Gently massage into rich lather.
3) Rinse thoroughly with warm water after 2~3 minutes.
1.For extenal use only. store at roomtemperature
2.Keep out of reach of children
3.when using this product
*use only as directed avoid cantact with eyes In case of accidental contact, rinse eyes with large amounts of cool water.
*Do not apply to wounds or damaged skin
4.Stop use and ask a doctor if
*condition worsen *redness is present *irriation develops
5. Other cautions when using the product
* If used continuously on one area, it may cause excessive scalp fat removal causing roughening of the scalp.
Humajor Co., Ltd.